Article contents
Economical Fe-doped Ta2O5 electrocatalyst toward efficient oxygen evolution: a combined experimental and first-principles study
Published online by Cambridge University Press: 03 August 2017
Abstract
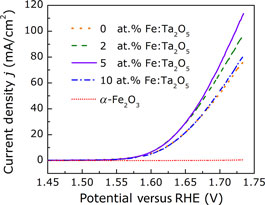
A non-precious metal catalytic system of Fe-doped Ta2O5 is developed by pulsed laser deposition toward efficient oxygen evolution reaction (OER). The optimal Fe concentration is determined to be 5 at.% for optimized OER activity via a series of electrochemical characterizations. The 5 at.% Fe-doped Ta2O5 nanolayer possesses a low onset overpotential of 0.22 V, an overpotential of 0.38 V at 10 mA/cm2 and a Tafel slope of 54 mV/dec. Comprehensive first-principles calculations attribute the enhanced OER activity to the substitutional FeTa dopants, which generate a new active OER site on surface and simultaneously accelerate electron transfer over oxygens.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 3
- Cited by




