Milk fat is an important determinant of milk nutritional quality. Certain SFA (mainly 12 : 0, 14 : 0 and 16 : 0) are considered to exert negative effects when consumed in excess, whereas others (4 : 0, anteiso-15 : 0, cis-9-18 : 1 and 18 : 3n-3) have potentially positive effects on human health(Reference Parodi1). For example, cis-9, trans-11, the major isomer of conjugated linoleic acid (CLA) in ruminant milk, exhibits anticarcinogenic and antiatherogenic properties in animal models(Reference Wahle, Heys and Rotondo2). Furthermore, it is well established that nutrition is a major factor that determines the concentration and secretion of specific fatty acids (FA) in ruminant milk(Reference Chilliard, Glasser and Ferlay3).
FA secreted in milk originate from de novo synthesis in the mammary gland and from the uptake of TAG and NEFA from arterial blood. Milk fat synthesis is known to involve the coordinated expression of several lipogenic genes(Reference Ollier, Robert-Granie and Bernard4), but the impact of nutrition on the regulation of mammary lipogenesis is not fully known(Reference Bernard, Leroux and Chilliard5). Indeed, studies on the nutritional regulation of mammary lipogenic gene expression in lactating cows have, in the main, involved investigations examining the response to diets containing high proportions of concentrates and/or plant oils and marine lipids(Reference Piperova, Teter and Bruckental6–Reference Harvatine and Bauman9). These experiments that induce milk fat depression (MFD) have provided evidence that specific biohydrogenation intermediates, namely the trans-FA isomers and mainly the trans-10 family formed during ruminal metabolism of dietary unsaturated FA, alter mammary lipid metabolism and gene expression(Reference Shingfield, Bernard and Leroux10). In addition, it has long been recognised that MFD in the bovine alters nutrient partitioning in favour of non-mammary tissues, adipose tissues in particular(Reference Bauman and Griinari11). These findings suggest that intermediary metabolism together with mammary lipogenesis may be altered by trans-FA isomers formed during the ruminal biohydrogenation of dietary unsaturated FA and/or that of other mechanisms, such as elevated ruminal propionate and plasma insulin concentration induced by high-concentrate diets, could also be involved.
In lactating goats, data on the role of nutrition in mammary metabolism and in the expression of genes encoding for key lipogenic enzymes are relatively scarce. Previous studies of these questions have only examined the effect of plant oils on either grass hay-based diets(Reference Bernard, Rouel and Leroux12–Reference Bernard, Leroux and Faulconnier14) or maize silage-based diets(Reference Bernard, Bonnet and Leroux15). Furthermore, indirect comparisons between the goat and the cow have identified species-specific differences in the responses of milk fat secretion and composition to high-concentrate diets rich in starch and supplemented with plant oils. In particular, these comparisons have demonstrated that MFD is absent in the goat(Reference Chilliard, Glasser and Ferlay3). The responses of both intermediary and mammary metabolism in the goat, a species that does not exhibit MFD in response to starch-rich diets supplemented with plant oil(Reference Bernard, Leroux and Chilliard5, Reference Shingfield, Bernard and Leroux10), are much lower in extent than the corresponding responses in the cow. However, the effect of variable dietary starch content and degradability has not been evaluated.
The present experiment examined the effect of the level of concentrate and the type of starchy concentrate on lipogenic gene expression in mammary and peripheral tissues during established lactation in goats. These investigations sought the following: (1) to understand possible mechanisms regulating mammary lipogenesis on diets containing different levels and types of starch and supplemented with plant oil and (2) to assess the possible contribution of adipose and liver tissues to changes in milk FA secretion. Sunflower-seed oil was selected as the source of 18 : 2n-6. The selected sources of starch were either maize grain or flattened wheat reported, respectively, as slowly and rapidly degradable sources of starch in the bovine(Reference Sauvant, Chapoutot and Archimède16) and caprine(Reference Archimède, Sauvant and Hervieu17). The study sought to test the following hypothesis: the level and source of starch in diets containing 18 : 2n-6 from sunflower-seed oil would increase specific trans intermediates formed in the rumen, resulting in different combinations of trans-11 and trans-10 isomers, with these isomers, in cows, being involved in different ways in the regulation of lipogenic gene expression, lipogenic enzyme activities and milk fat secretion(Reference Harvatine and Bauman9). The subsequent hypothesis was that the mammary lipid synthesis and intermediary lipid metabolism could be modified by the type of starchy concentrate with such modifications that could result from possible variations in trans-FA synthesised in the rumen and/or from possible variations in the concentrations of plasma insulin and metabolites.
Materials and methods
Animals and diets
All experimental procedures were approved by the Animal Care Committee of INRA in accordance with the ‘Use of Vertebrates for Scientific Purposes Act’ 1985. The animals were recruited to experiments and allocated to treatment groups according to milk yield, milk fat and protein content, parity, stage of lactation and genotype score at the αS1-casein locus. Goats of the ‘middle-type’ αS1-casein genotype content were used to avoid any effects on milk traits(Reference Grosclaude, Ricardeau and Martin18) or FA composition(Reference Chilliard, Rouel and Leroux19). A total of fifteen multiparous (3·1 (sd 1·2) lactations) Alpine goats in mid-lactation (91 (sd 7·7) d in lactation) were given three experimental diets according to a replicated 3 × 3 Latin square design with 21 d experimental periods, with five groups of three animals each. From the fifteen goats recruited, one animal was withdrawn from the experiment due to mammary cysts. Each experimental period consisted of a 14 d adaptation to treatments (which was sufficient to achieve a full response of milk FA secretion in goats)(Reference Chilliard, Glasser and Ferlay3) and a 7 d sampling period. Goats were housed in a metabolism unit in individual stalls. The animals were allowed continuous access to water and were milked at 08.00 and 16.00 hours. Experimental diets were formulated to meet energy and protein requirements(Reference Jarrige20). The three diets were offered as two equal meals at 08.30 and 16.30 hours. Diets were comprised of natural grassland hay that was offered ad libitum, with either a high level of forage (0·8 kg/d) and supplemented with 130 g/d sunflower-seed oil (F; 6·9 % of diet DM; sunflower-seed oil from Auvergne Trituration, Lezoux, France), or a high level of concentrate (1·4 kg/d) with maize grain (CM; 1 kg/d; 5·4 % of diet DM) or flattened wheat (CW; 1 kg/d; 5·5 % of diet DM) as source of starch and supplemented with 130 g/d sunflower-seed oil.
Measurements and sampling
Individual intake was recorded daily. However, only the measurements collected during the last week of each experimental period were used for statistical analysis. During each experimental period, representative samples of hay, maize grain, flattened wheat and concentrates were composited daily and stored at − 20°C. Chemical composition of feed ingredients was determined using standard procedures(21). Milk yields of individual goats were recorded three times per week. However, only the measurements collected during the last week of each experimental period were analysed statistically. Samples of milk were collected from all goats over four consecutive milkings starting at 08.00 hours on day 19 of each experimental period for fat, crude protein and lactose analysis. Milk fat, crude protein and lactose were determined in samples treated with preservative (potassium bichromate; Merck, Fontenay-Sous-Bois, France) by mid-infrared spectroscopy(21). Unpreserved samples of milk were also collected over two consecutive milkings starting at 08.00 hours on day 20 of each experimental period, stored at − 20°C, composited according to yield and analysed for the determination of FA composition(Reference Bernard, Shingfield and Rouel22). The live weight of each experimental animal was measured at the start and end of each experimental period.
Blood samples were collected on day 20 of each experimental period at 07.30 hours. Samples from the jugular vein were collected into evacuated collection tubes (Venoject; C.M.L., Nemours, France) containing potassium ethylene diamine tetra-acetic acid. Once collected, blood samples were centrifuged (1500 g for 15 min at 4°C, stored at − 20°C), and plasma was analysed for insulin and metabolite concentrations(Reference Bernard, Rouel and Leroux12).
On day 21 of periods 1 and 2, mammary biopsies were obtained after morning milking for tissue RNA extraction using sterile Trucut needles (CE Sherwood Medical, Ballymoney, Northern Ireland) according to the manufacturer's instructions. Approximately 20–25 mg of tissue were taken, rinsed in 0·9 % saline sterile solution, inspected to verify tissue homogeneity and snap-frozen in liquid N2. Samples were stored at − 80°C before RNA extraction. Collection of tissue biopsies resulted in minimal bleeding, and milk appeared normal after one to three subsequent milkings. During this period, extreme care was taken during manual milking to remove possible blood clots lodged in the glands. No intra-mammary infections or loss of milk production were encountered following mammary tissue biopsies.
At the end of the experiment, corresponding to days 26 and 27 of the last experimental period (period 3), goats were slaughtered after morning milking. Immediately before slaughter, animals were milked to remove most of the milk in the mammary glands. Immediately after death, samples of mammary, liver, and perirenal and omental adipose tissues were collected under sterile conditions, frozen in liquid N2 and stored at − 80°C for RNA extraction and enzyme assays. Samples of mammary tissue were analysed for stearoyl-CoA desaturase (SCD) activity immediately after collection. Samples of perirenal and omental adipose tissues were also collected immediately after slaughter, stored at 37°C, fixed with osmium tetra-oxide and isolated in 8 m-urea for the determination of adipocyte volume(Reference Chilliard, Gagliostro and Flechet23).
Enzyme activities and quantification of mRNA abundances by quantitative PCR
The enzyme activities and/or mRNA abundance of enzymes involved in the major metabolic pathways of lipogenesis, in particular the uptake and transport of FA, de novo FA synthesis and TAG synthesis, were measured in mammary, liver, perirenal and omental adipose tissues.
The following lipogenic enzyme activities were assayed in samples of mammary and/or of perirenal and omental adipose tissues: lipoprotein lipase (LPL; EC 3.1.1.34), an enzyme involved in the uptake of FA from circulating TAG(Reference Faulconnier, Thevenet and Flechet24); fatty acid synthase (FAS; EC 2.3.1.85), acetyl-CoA carboxylase (ACC; EC 6.4.1.2), malic enzyme (ME; EC 1.1.1.40) and glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49), involved in de novo lipogenesis; glycerol-3-phosphate dehydrogenase (G3PDH; EC 1.1.1.8), involved in FA esterification(Reference Chilliard, Gagliostro and Flechet23); SCD (EC 1.14.99.5), involved in the Δ9 desaturation of FA(Reference Bernard, Rouel and Leroux12).
The mRNA abundance of the following lipogenic genes was also measured in (1) samples of mammary and/or perirenal and omental adipose and/or liver tissues: ACACA, FASN, SCD1 and LPL; platelet glycoprotein 4 (CD36) and fatty acid-binding proteins in heart (FABP3) and adipocyte (FABP4) involved in the uptake and intracellular transport of FA; 1-acyl-sn-glycerol-3-phosphate acyltransferase α (AGPAT1) and diacylglycerol acyltransferase 1 (DGAT1), involved in the esterification of FA to glycerol; (2) samples of mammary tissues: xanthine dehydrogenase/oxidase (XDH), butyrophilin subfamily 1 member A1 (BTN1A1), glycosylation-dependent cell adhesion molecule 1 (GLYCAM1), lactadherin (MFGE8) and mucin-1 (MUC1), which are the major proteins of the milk fat globule (MFG) membrane; (3) samples of liver tissues: the short (ObRa) and long form (ObRb) of the leptin receptor involved in the signal transduction of the effect of leptin(Reference Chelikani, Glimm and Kennelly25), which is known to regulate SCD expression in part.
Total RNA was isolated from samples of mammary tissue using the RNeasy mini kit (QIAGEN, Inc., Courtaboeuf, France) and from samples of liver using the NucleoSpin RNA II kit (Macherey Nagel, Inc., Bethlehem, PA, USA). Total RNA extraction of perirenal and omental adipose tissues was carried out as described previously(Reference Bonnet, Leroux and Faulconnier26). DNA contamination was removed by DNase I treatment (Amplification grade; Invitrogen, Life Technologies, Carlsbad, CA, USA) before complementary DNA synthesis. Integrity and concentration of RNA and RT-PCR were assessed as described previously(Reference Bernard, Rouel and Leroux12). mRNA abundances of FASN, ACACA, LPL, SCD1, AGPAT1 and DGAT1, plus the housekeeping gene peptidyl-prolyl cis–trans isomerase A (PPIA) or cyclophilin A were quantified in duplicate by real-time quantitative RT-PCR using fluorescent TaqMan probes (Applied Biosystem, Warrington, UK) and a LightCycler System (Roche Molecular Biochemicals, Indianapolis, IN, USA) using specific primers and probes (Table 1). The abundances of FABP3, FABP4, XDH, BTN1A1, MFGE8, MUC1, ObRa and ObRb mRNA were quantified in duplicate by RT-PCR using the fluorescent SYBR Green I methodology (Applied Biosystem) and specific primers reported in Table 1. The abundance of targeted gene transcripts was expressed as the mRNA copy number relative to PPIA (housekeeping gene) to account for variations in RNA integrity, RNA quantification and complementary DNA synthesis.
Table 1 Primer and probe sequences and conditions used for real-time RT-PCR

T, PCR annealing temperature; F, forward primer; R, reverse primer; P, Taqman probe.
PCR efficiency was 95·6 (sd 9·5) % for the fourteen target genes and 95·5 (sd 1·5) % for PPIA.
Statistical analysis
Measurements of DM intake, milk production, milk composition, FA secretion in milk, mammary mRNA abundance and plasma metabolite concentrations were subjected to ANOVA for a 3 × 3 Latin square design using the General Linear Models Procedure of SAS (SAS Institute, Cary, NC, USA) with a model that included the effects of treatment, period and goat. Treatment means were compared using the least-square means procedure (SAS Institute), with differences declared significant at P < 0·05. Measurements of enzyme activity and mRNA abundance in samples of mammary, liver, and perirenal and omental adipose tissues collected at slaughter were evaluated statistically using the non-parametric Wilcoxon U test due to the small set of data (n < 6) and high individual variability. Treatment effects were considered significant at P < 0·05.
Results
Diet composition
The crude protein, neutral-detergent fibre and acid-detergent fibre content of the diets are described in Table 2. The starch content of the two high-concentrate diets (CM and CW) was 336 and 318 g/kg DM, respectively, compared with 164 g/kg DM for the F diet. The mean forage:concentrate ratio (on a DM basis) for the F, CM and CW diets was 57:43, 37:63 and 31:69, respectively. The goats consumed 145, 147 and 123 g/d of total C18-FA and 318, 749 and 655 g/d of starch for the F, CM and CW diets, respectively (Table 3).
Table 2 Ingredient and chemical composition of the experimental diets
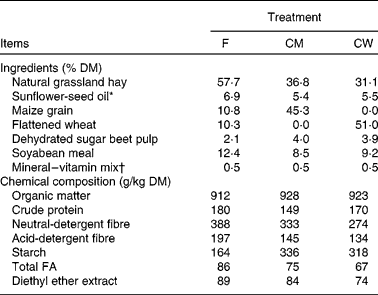
F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil; FA, fatty acids.
* Sunflower-seed oil contained (g/100 g FA) 16 : 0 (6·8), 18 : 0 (3·8) cis-9-18 : 1 (30·5), 18 : 2n-6 (57·9) and 22 : 0 (0·3).
† Mineral–vitamin supplement (Usine d'Ussel, Murat, France) contained (g/kg): Ca, 240; P, 60; Mg, 50; Na, 15; Zn, 7; Mn, 6; dl-α-tocopherol, 0·3; retinol, 0·2; cholecalciferol, 0·002.
Table 3 Effect of the level and type of starchy concentrate in diets supplemented with sunflower-seed oil on DM intake, milk yield, milk composition, and calculated energy and protein balance in goats
(Mean values with their standard errors, n 42; error df=24)

F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil; FA, fatty acids; NEl, net energy of lactation; PDI, digestible protein at the intestine.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Energy and protein balances calculated according to INRA(Reference Jarrige20).
Animal performance
Even though the consumption of concentrate rich in maize grain increased the DM intake (P = 0·003), the calculated energy and N balance were positive for all the experimental treatments (Table 3). The calculated energy balance increased (P < 0·001) with the CM and CW diets, compared with the F diet (Table 3). There were no changes in body weight according to treatments (results not shown), which is not surprising since the length of the experimental periods was only 3 weeks. Relative to the F diet, the CM and CW diets enhanced milk yield (+500 and +620 g/d, respectively; P < 0·001), milk protein yield (+19 and +22 g/d, respectively; P = 0·04) and milk lactose yield (+25 and +31 g/d, respectively; P < 0·001) without changing milk fat yield. Conversely, relative to the F diet, the CM and CW diets decreased milk fat content ( − 4·1 and − 4·4 g/kg, respectively; P = 0·003), whereas no effect (P>0·05) was observed for milk protein and lactose content (Table 3).
Plasma metabolites
Both high-concentrate diets increased plasma glucose concentrations, whereas plasma insulin concentration increased only when the concentrate was rich in maize grain (CM; Table 4). Compared with the F diet, the CM diet decreased plasma NEFA concentrations. Furthermore, the CM diet increased plasma acetate, whereas no effect was observed on β-hydroxybutyrate content (Table 4).
Table 4 Effect of the level and type of starchy concentrate in diets supplemented with sunflower-seed oil on plasma insulin and metabolite concentrations in goats
(Mean values with their standard errors, n 42; error df=24)

F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Mammary, adipose tissue and liver lipid metabolism
Dietary treatments had no effect (P>0·05) on the abundance of mRNA in mammary tissue encoding for lipogenic genes (LPL, ACACA, FASN, SCD1, AGPAT1, DGAT1, CD36, FABP4 and FABP3) and for MFG proteins (XDH, BTN1A1, GLYCAM1, MFGE8 and MUC1) (Table 5). However, a significant (P = 0·01) and close (R>+0·60) inter-individual relationship between the mRNA abundances was observed only for the following five pairs of genes: ACACA and FASN, BTN1A1 and XDH, XDH and DGAT1, DGAT1 and FABP4, SCD1 and AGPAT1 (Fig. 1).
Table 5 Effect of the level and type of starchy concentrate in diets supplemented with sunflower-seed oil on the mRNA abundance of lipogenic genes in the mammary tissue of goats
(Mean values with their standard errors, n 42; error df=24)

F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil; LSD, least significance difference; LPL, lipoprotein lipase; ACACA, acetyl-CoA carboxylase; FASN, fatty acid synthase; SCD1, stearoyl-CoA desaturase; AGPAT1, 1-acyl-sn-glycerol-3-phosphate acyltransferase α; DGAT1, diacylglycerol acyltransferase 1; CD36, platelet glycoprotein 4; XDH, xanthine dehydrogenase/oxidase; BTN1A1, butyrophilin subfamily 1 member A1; GLYCAM1, glycosylation-dependent cell adhesion molecule 1; MFGE8, lactadherin; MUC1, mucin-1; FABP4, fatty acid-binding protein, adipocyte; FABP3, fatty acid-binding protein, heart.
* mRNA levels expressed in arbitrary units determined as abundance relative to cyclophylin A mRNA and multiplied by 100.
† LSD for comparison with observed non-significant differences.

Fig. 1 Relationships between mRNA abundances of (a) acetyl-CoA carboxylase (ACACA) and fatty acid synthase (FASN): y = − 0·0028x 2+0·0999x+0·152; r +0·72, n 42, P = 0·01; (b) xanthine oxidoreductase (XDH) and butyrophilin (BTN1A1): y = 2·682x+3·064; r +0·84, n 42, P = 0·01; (c) diacylglycerol acyltransferase 1 (DGAT1) and XDH: y = 2·405x+1·358; r +0·73, n 42, P = 0·01; (d) DGAT1 and adipocyte fatty acid-binding protein (FABP4): y = 2·014x+0·150; r +0·71, n 42, P = 0·01 and (e) 1-acyl-sn-glycerol-3-phosphate acyltransferase α (AGPAT1) and stearoyl-CoA desaturase (SCD1): y = 2·167x+3·893; r +0·60, n 42, P = 0·01 in the mammary gland of goats fed a diet with a high level of forage and supplemented with sunflower-seed oil (○) or a diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil (△), or a diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil (□). The mRNA abundances are expressed in arbitrary units, determined as abundance relative to cyclophylin A mRNA and multiplied by 100.
In perirenal and omental adipose tissues, the treatments had no effect (P>0·05) on the abundance of mRNA encoding for LPL, FASN, ACACA and AGPAT1 (Table 6), except for a decrease in FASN mRNA and an increase in AGPAT1 mRNA for the CM diet in omental adipose tissue. In the liver, SCD1 mRNA increased with the CM diet (Table 6). Furthermore, dietary treatments had no effect (P>0·05) on LPL, FAS, G6PDH, ME and G3PDH activities in perirenal and omental adipose tissues (Table 7), as well as in mammary tissues, with the exception of a decrease (P < 0·05) in G3PDH for the CM diet compared with the CW diet. SCD and ACC activities in mammary tissues were not affected by the dietary treatments (Table 7).
Table 6 Effect of the level and type of starchy concentrate in diets supplemented with sunflower-seed oil on the mRNA abundance of genes involved in lipid metabolism in perirenal and omental adipose and liver tissues of goats
(Mean values with their standard errors)

F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil; LPL, lipoprotein lipase; FASN, fatty acid synthase; SCD1, stearoyl-CoA desaturase; AGPAT1, 1-acyl-sn-glycerol-3-phosphate acyltransferase α; ACACA, acetyl-CoA carboxylase; ObRa, leptin receptor isoform a; ObRb, leptin receptor isoform b.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* mRNA levels expressed in arbitrary units determined as abundance relative to cyclophylin A mRNA and multiplied by 100.
† Mean value was significantly different from the CM diet (P < 0·10).
Table 7 Effect of the level and type of starchy concentrate in diets supplemented with sunflower-seed oil on lipoprotein lipase (LPL), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), glucose-6-phosphate dehydrogenase (G6PDH), malic enzyme (ME), stearoyl-CoA desaturase (SCD) and glycerol-3-phosphate dehydrogenase (G3PDH) activities in the mammary, perirenal and omental adipose tissues of goats
(Mean values with their standard errors)

F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Adipocyte volume determined in samples collected at slaughter, maintained at 37°C, fixed with osmium tetra-oxide and isolated in 8 m-urea.
Milk fatty acid secretion
A high level of concentrate in the diet increased (P < 0·001) the milk secretion of C6- to C14-SFA and cis-9-14 : 1 and 16 : 0 secretion. These increases were associated with a decrease (P < 0·001) in the output of trans-9-16 : 1, trans-9,11-18 : 1 and cis-9, trans-11-18 : 2 in milk (Table 8). Relative to the F diet, decreases in the milk fat trans-11-18 : 1 and cis-9, trans-11-18 : 2 secretions were − 67 and − 63 %, respectively, for the CW diet and − 32 and − 36 %, respectively, for the CM diet. Relative to the F diet, the milk fat medium-chain FA (C10- to C16-SFA) and cis-9-14 : 1 secretions were higher, and higher for the CW diet than for the CM diet (Table 8). The inclusion of flattened wheat as a type of starch in the high-concentrate diet (CW) resulted in increases (P < 0·05) in milk trans-10-18 : 1, 18 : 2n-6 and 18 : 3n-3 output and decreases (P < 0·05) in milk cis-9-18 : 1 and Σ C18 output, compared with the CM and F diets (Table 8).
Table 8 Effect of the level and type of starchy concentrate in diets supplemented with sunflower-seed oil on the secretion of major fatty acids (FA) in milk and milk fat Δ-9 desaturase ratios in goats
(Mean values with their standard errors, n 42; error df=24)

F, diet with a high level of forage and supplemented with sunflower-seed oil; CM, diet with a high level of concentrate with maize grain as source of starch and supplemented with sunflower-seed oil; CW, diet with a high level of concentrate with flattened wheat as source of starch and supplemented with sunflower-seed oil; CLA, conjugated linoleic acid.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
The consumption of the CM or CW diet had no significant effect (P>0·15) on milk fat Δ9 desaturase ratios (cis-9-14 : 1:14: 0, cis-9-16 : 1:16 : 0 and cis-9-18 : 1:18 : 0) except for an increase in the cis-9, trans-11-CLA:trans-11-18 : 1 concentration ratio for the CW diet, compared with the F and CM diets (Table 8).
Discussion
DM intake and milk production and composition
In goats, the observed milk and protein yield increases in response to the addition of starchy concentrate to a lipid-rich diet (Table 3) are consistent with the results of previous studies(Reference Chilliard, Glasser and Ferlay3, Reference Andrade and Schmidely27). The increase in milk yield in response to the addition of starchy concentrate was associated with (1) a higher yield of lactose in milk in agreement with previous studies(Reference Andrade and Schmidely27), consistent with the role of lactose in the regulation of milk osmolarity(Reference Cant, DePeters and Baldwin28) and (2) the increased DM intake and energy intake (results not shown) for the CM diet.
The increase in dietary concentrate and starch content in the presence of sunflower-seed oil in the present experiment had no effect on milk fat yield, whereas it decreased milk fat concentration, probably owing to a dilution effect. In contrast, in cows fed diets supplemented with linseed oil(Reference Loor, Ferlay and Ollier29) or ‘corn oil’(Reference Griinari, Dwyer and McGuire30), increasing the level of dietary concentrate from 36 to 66 %(Reference Loor, Ferlay and Ollier29) and from 50 to 80 %(Reference Griinari, Dwyer and McGuire30) decreased the milk fat yield by 42 and 55 %, respectively. In goats, increasing the percentage of concentrate in a lipid-supplemented diet up to 69 % (present study), 65 %(Reference Andrade and Schmidely27) or 68 %(Reference Chilliard, Glasser and Ferlay3) did not change or increased milk fat secretion. Otherwise, in cows, feeding starch-based diets that differed by the source of starch (cracked wheat v. potatoes) showed that the quicker ruminal degradation of wheat starch was associated with a decrease in milk fat(Reference Jurjanz, Monteils and Juaneda31). Altogether these results highlight the differences between ruminant species in ruminal kinetics or metabolism and in the regulation of mammary lipid metabolism. The mechanisms underlying this species difference remain largely unknown.
Milk fatty acid secretion and mammary metabolism
In the present experiment, the increased output of milk medium-chain FA (10 : 0 to 16 : 0;+25 % with the CM diet and +45 % with the CW diet) with a high level of concentrate in lipid-rich diets was not accompanied by significant alterations in ACC, FAS, G6PDH, ME or G3PDH activities, nor by significant alterations in ACACA and FASN mRNA abundances. These findings can be compared with previous studies in goats on response to plant lipids(Reference Bernard, Rouel and Leroux12, Reference Bernard, Bonnet and Leroux15) where decreases in milk medium-chain FA secretion ( − 18 to − 27 %) were not accompanied by changes in mammary lipogenic gene expression or activity. Altogether these data suggest that, in the caprine, changes in milk medium-chain FA in response to dietary treatments are not mediated by changes in lipogenic gene expression or ex vivo enzyme activity.
The secretion of long-chain FA (Σ C18) in response to the addition of starch to the diet was maintained (CM) or slightly decreased (CW) (Table 8). These low variations in milk C18-FA secretion were not accompanied by variations in mammary LPL mRNA abundance and/or activity, or in mRNA abundance of genes involved in the uptake, transport and trafficking of FA in the cells (CD36, FABP3 and FABP4).
Otherwise, an absence of variation of mammary mRNA abundance for genes involved in the desaturation of FA (SCD1) or in TAG synthesis (AGPAT1 and DGAT1) following the increase of concentrate in the diet is in agreement with findings on SCD1 in previous studies. These studies have demonstrated that SCD1 gene expression varies little, except when supplements rich in n-3 FA are added to the diet in the cow(Reference Ahnadi, Beswick and Delbecchi7) and the goat(Reference Bernard, Rouel and Leroux12), thereby down-regulating SCD1.
In the present experiment, no alteration of mammary mRNA abundance for genes coding for the major proteins of the MFG membrane (BTN1A1, XDH, MFGE8 and MUC1) was observed in response to the concentrate. This result is consistent with the observed absence of milk fat yield modification. However, a positive inter-individual relationship between BTN1A1 and XDH (r +0·84, P < 0·01; Fig. 1(b)) was observed. This observation suggests the possible co-regulation of these two genes. This hypothetical co-regulation is supported by the model of secretion of MFG(Reference Mather and Keenan32). This model supposes that milk secretion involves a tripartite complex having the following components: the integral transmembrane protein butyroplylin; the cytoplasmic enzyme xanthine oxidoreductase (XDH); the lipid droplet surface protein adipophilin. Overall, these results are consistent with the idea that the expression of proteins of the MFG membrane in the mammary gland is closely related to secretory activity(Reference McManaman, Palmer and Wright33).
The present study characterised the mammary expression of fourteen genes involved in the different pathways of lipogenesis, as well as in the protein of the MFG membrane. This the first study to characterise the mammary expression of these genes in goats. The results of the study also demonstrated positive associations between the following pairs of genes: ACACA and FASN mRNA (r +0·72, P < 0·01; Fig. 1(a)), as described previously(Reference Bernard, Leroux and Chilliard5), SCD1 and AGPAT1 mRNA (r +0·60, P < 0·01; Fig. 1(e)), DGAT1 and FABP4 mRNA (r +0·71, P < 0·01; Fig. 1(d)) and XDH and DGAT1 mRNA (r +0·73, P < 0·01; Fig. 1(c)). Such relationships probably result from shared regulation by the same transcription factor or from concomitant regulation by different transcription factors(Reference Harvatine and Bauman9) for these genes or other regulatory mechanisms involving microRNA(Reference Lynn34).
In the present study, addition of concentrate (+20–25 % in the diet) and starch (+16 % of the DM intake) in the diet induced increases in milk medium-chain FA (10 : 0 to 16 : 0) output in goats (+25 and +45 %, respectively, for the CM and CW diets), compared with a reduction of 31 %(Reference Loor, Ferlay and Ollier29) or 32 %(Reference Griinari, Dwyer and McGuire30) in cows offered diets with comparable increases in concentrate (+30 % in the diet(Reference Loor, Ferlay and Ollier29, Reference Griinari, Dwyer and McGuire30)) and starch content (+15 %(Reference Loor, Ferlay and Ollier29) and +16 % DM(Reference Griinari, Dwyer and McGuire30)) and supplemented with linseed oil(Reference Loor, Ferlay and Ollier29)or ‘corn oil’(Reference Griinari, Dwyer and McGuire30) (rich in 18 : 2n-6 and 18 : 1n-9). In addition, whereas no or slight variation of Σ C18 secretion was observed in goats (Table 8), cows fed similar diets exhibited a reduction of 53 %(Reference Loor, Ferlay and Ollier29) or 43 %(Reference Griinari, Dwyer and McGuire30) of Σ C18 secretion. These findings are in agreement with the lower inhibitory effect of dietary trans-10, cis-12-CLA on the uptake and incorporation of long-chain FA in goats than in cows(Reference Shingfield, Rouel and Chilliard35). However, direct comparisons of ruminant species fed the same diet are required to confirm these observations.
The observed differences between ruminant species in response to a high concentrate added to lipid-rich diets might be related to the following: (1) the synthesis of specific ruminal biohydrogenation intermediates known to exert anti-lipogenic effects; (2) species specificities in mammary lipid metabolism; and/or (3) differences in acetate and β-hydroxybutyrate supply for de novo lipid synthesis in the mammary gland.
Studies on the nutritional regulation of mammary lipogenic gene expression and enzyme activities in response to MFD diets in the lactating cow have pointed out that specific ruminal biohydrogenation intermediates may exert mammary anti-lipogenic effects in the bovine(Reference Shingfield, Bernard and Leroux10). Those intermediates are associated with the ruminal trans-10 pathway, particularly with trans-10, cis-12-CLA that has been shown unequivocally to inhibit milk fat synthesis in ruminants (but to a lower extent in goats than in cows)(Reference Shingfield, Rouel and Chilliard35), although other biohydrogenation intermediates may also be involved as suggested for the cis-10, trans-12-CLA, trans-9, cis-11-CLA and trans-10-18 : 1(Reference Shingfield, Bernard and Leroux10). In the present experiment, mammary secretion of trans-10-18 : 1 attained its maximal value in response to the CW treatment and corresponded to a milk concentration of 2·17 % of total FA (results not shown). This result is in the same range as the values observed in milk from cows fed a comparable diet (2·84 %(Reference Loor, Ferlay and Ollier29) and 2·90 %(Reference Griinari, Dwyer and McGuire30) of total FA) that induced a MFD. This indirect comparison illustrates the marked differences between goats and cows in the relationship between changes in milk fat secretion and increases in milk fat trans-10-18 : 1 concentration. Indeed, it has been shown that within the range of milk trans-10-18 : 1 concentrations observed in goats (0–5·2 % of total FA), almost all milk fat yield responses are positive, whereas the converse is true in cows(Reference Bernard, Leroux and Chilliard5, Reference Shingfield, Bernard and Leroux10). In the present study, an increase in trans-10-18 : 1 (from 0·88 to 2·17 % of total FA for the F and CW diets, respectively) was accompanied by an increase in milk medium-chain FA secretion (C10–C16,+45 %). These results suggest that trans-10-18 : 1 and, in general, the FA family associated with the trans-10 pathway are much less potent in caprine mammary cells in agreement with results from a study in goats receiving dietary supplements of trans-10, cis-12-CLA(Reference Shingfield, Rouel and Chilliard35). This finding contrasts with the observed anti-lipogenic activity of these compounds in the cow.
The present study also investigated the substrate supply for mammary de novo lipid synthesis. The study revealed increased or stable plasma acetate and 3-hydroxy-butyrate concentrations in response to dietary concentrate. Decreases in plasma acetate and 3-hydroxy-butyrate concentrations(Reference Loor, Ferlay and Ollier29) and decreases in acetate or stable butyrate concentrations in ruminal fluid(Reference Griinari, Dwyer and McGuire30) have been observed in cows fed comparable diets. These differences between goats and cows may contribute to the increase in de novo FA synthesis in the mammary gland in goats. Moreover, these results suggest differences in the ruminal metabolism of dietary carbohydrate among ruminant species. They imply a greater stability of ruminal fermentation processes in response to various dietary conditions in goats, compared with cows(Reference Bernard, Shingfield and Rouel22). Accordingly, in goats fed diets with a high level of concentrate, the nature of starchy concentrate (slowly or rapidly degradable starch) had little or no influence on the rumen fermentation parameters(Reference Archimède, Sauvant and Hervieu17) (stable and high rumen pH, volatile FA profile) compared with studies from bovine(Reference Jurjanz, Monteils and Juaneda31). These differences among ruminant species could be attributed to differences in feeding behaviour(Reference Goetsch, Gipson and Askar36): (1) a greater ingestive chewing efficiency of the goats that may explain the smaller differences between maize and wheat starch and (2) greater eating and rumination times for goats than for sheep and cows with high-concentrate diets which have been related to higher rumen-buffering capacity in goats(Reference Desnoyers, Giger-Reverdin and Sauvant37).
The present experiment demonstrated that switching from a low- to high-concentrate diet in goats receiving 18 : 2n-6 from sunflower-seed oil does not induce MFD or variations in mammary lipogenic expression, irrespective of the source of starch in concentrate supplements.
Adipose and liver lipid metabolism
The present experiment found no changes in lipogenic gene expression and/or enzyme activity in perirenal and omental adipose tissues in response to concentrate in a lipid-rich diet. This result is in agreement with the lack of change found in milk fat secretion. In cows, high-concentrate diets have been found to induce MFD, which involves changes in biohydrogenation pathways in the rumen leading to increased level of numerous milk biohydrogenation intermediates including trans-10, cis-12-18 : 2. This effect was associated with less energy used for milk fat synthesis and an alteration of nutrient partitioning in favour of non-mammary tissues, particularly adipose tissues(Reference Bauman and Griinari11, Reference Griinari, Bauman, Sejrsen, Hvelplund and Nielsen38), which was accompanied by the increased transcription of genes involved in lipogenesis in subcutaneous adipose tissue(Reference Harvatine, Perfield and Bauman39). The mechanisms of these differences between the responses of goat and cow adipose tissues to a high-concentrate diet remain to be unravelled but could be related to differences in plasma insulin concentrations or in the synthesis of specific ruminal biohydrogenation intermediates, as discussed in the previous section.
Lipogenic activities in adipose tissue were not altered in response to the increased level of concentrate in the diets. Indeed, the absence of change in these lipogenic activities occurred despite the slightly higher plasma insulin and glucose concentrations and calculated energy balance that are known to be involved in the regulation of adipose tissue lipogenesis(Reference Vernon40). However, it is likely that the significantly elevated calculated energy balance and plasma glucose and insulin concentrations observed for the CM diet may have contributed to the inhibition of the lipolytic pathways in adipose tissue(Reference Bauman, Elliot and Mepham41, Reference Gagliostro, Chilliard and Davicco42), as suggested by the lower plasma NEFA concentration found with this diet, without being sufficient to induce lipogenesis.
Comparison of enzyme activities between tissues (Table 7) highlights their specific lipid metabolism. The study found a substantially greater activity of G3PDH and ME in perirenal and omental adipose tissues compared with the mammary gland, whereas the activities of LPL, FAS and G6PDH were similar between tissues. Those results are in line with previous data for perirenal adipose tissue and mammary glands in goats fed maize silage-based diets supplemented with plant oils(Reference Bernard, Bonnet and Leroux15).
In the liver, addition of concentrate in the lipid-rich diets had no effect on ACACA and SCD1 mRNA abundance, whereas the CW diet significantly decreased SCD1 mRNA abundance, compared with the CM diet. This observation, together with the higher leptinaemia reported with the CW treatment(Reference Bonnet, Delavaud and Bernard43), is in agreement with data from rodents demonstrating that leptin suppresses SCD1 transcription and activity in the liver(Reference Biddinger, Miyazaki and Boucher44). This effect may be facilitated by the observed tendency (P = 0·08) towards a higher expression of the leptin receptor isoform ObRa in the CW treatment, compared with the CM treatment. This leptin receptor isoform mRNA has been characterised in the bovine and is highly expressed in the liver and spleen(Reference Chelikani, Glimm and Kennelly25, Reference Kawachi, Yang and Hamano45). This isoform receptor could be involved in leptin transport, thereby contributing to the multiple physiological functions of leptin in ruminants(Reference Wylie46). To our knowledge, such relationships have not yet been reported for caprine liver. However, the role of circulating levels of leptin in the regulation of lipid metabolism in adipose and other tissues in the lactating ruminant remains unclear and needs to be clarified(Reference Chilliard, Delavaud and Bonnet47).
Conclusions
Increases in the secretion of medium-chain FA in response to the level and the type of starchy concentrate added to a lipid-rich diet in the goat appear to be regulated by mechanisms independent of changes in mammary lipogenic gene expression. This response in goats differs from that observed in cows. This difference between goats and cows were discussed according to the following: (1) a lower sensitivity of the mammary gland to the anti-lipogenic activity of specific trans-10 FA biohydrogenation intermediates and/or (2) increased or more stable acetate and butyrate availability which could putatively increase de novo FA synthesis in the goat mammary gland, compared with decreased availability in the cow, with these changes being probably related to different feeding behaviour. In addition, the concentrate had no effect on lipogenic gene expression and/or activity in perirenal and omental adipose and liver tissues except SCD1 mRNA and a trend for ObRa in the liver. This finding is in agreement with the absence of a response of milk fat secretion to the concentrate in goats. Despite the absence of variation in the mammary gene expression of lipogenic enzymes and proteins of the MFG membrane, the data of the present study revealed positive inter-individual correlations between few pairs of genes which would need further investigations.
Acknowledgements
This study was supported by European funding under the project EU BIOCLA (project QLK1-2002-02362). The authors gratefully acknowledge the staff of the Animal Nutrition and Metabolism Unit Les Cèdres, in particular André Combeau and Christophe Mathevon, for diligent care of the experimental animals, Roland Jailler and his team for assisting in the slaughter of the experimental animals, Philippe Gaydier and Denys Durand for the collection of the mammary biopsies, Sébastien Bes, Cyril Labonne, Pierre Capitan, Martine Tourret, Didier Bany and Cecilia Sosa for assistance with the analysis of the samples and Frédéric Glasser for advice on statistical analyses. All authors have contributed to the preparation of the paper and agree with the submitted manuscript content. L. B. and Y. C. designed the research. L. B. and J. R. performed the research, and L. B. and M. B. analysed the samples. L. B., Y. C., J. R., M. B. and C. L. analysed the data and drafted the paper. There are no conflicts of interest.













