Introduction
The zona pellucida (ZP) is an envelope that contains a species-dependent sperm receptor, it triggers the acrosome reaction and in the frame of a physiological process after fertilization undergoes biochemical modifications called zona hardening that prevent polyspermy (Cohen, Reference Cohen, Elder and Cohen2007). The ZP ruptures at the blastocyst stage in a process called hatching and this event allows embryo implantation. Mechanical expansion and contraction/elasticity of the blastocyst and, furthermore, thinning of the ZP are all involved in hatching and are essential for this process (Cohen, Reference Cohen, Elder and Cohen2007). Both elasticity/zona hardening and ZP thinning can be influenced adversely by advancing maternal age and in vitro culture conditions (Cohen et al., Reference Cohen, Alikani, Trowbridge and Rosenwaks1992; Huisman et al., Reference Huisman, Fauser, Eijkemans and Pieters2000). Data indicate that zona hardening may be initiated by cryopreservation as well. (Cohen et al., Reference Cohen, Lindheim and Sauer1999; Elhussieny et al., Reference Elhussieny, El Mandoub, Hanafi, Mansour and El-Kotb2013).
Abnormalities in the hatching have been suggested as a possible explanation for the low implantation rates observed in some patients, in advanced maternal age and in frozen–thawed IVF/ICSI-ET cycles (Cohen, Reference Cohen, Elder and Cohen2007). Assisted hatching (AH) procedures are based on creating an artificial opening or thinning ZP, which is believed to support hatching and implantation of embryos that are otherwise unable to escape from the ZP (Cohen et al., Reference Cohen, Alikani, Trowbridge and Rosenwaks1992). Yet, the effectiveness of AH to improve the outcome of fresh and frozen IVF/ICSI-ET cycles remains controversial (Ng et al., Reference Ng, Naveed, Lau, Yeung, Chan, Tang and Ho2005; Ge et al., Reference Ge, Zhou, Zhang and Lin2008; Lan et al., Reference Lan, Huang, Lin, Kung and Chang2009; Carney et al., Reference Carney, Das, Blake, Farquhar, Seif and Nelson2012; Razi et al., Reference Razi, Halvaei and Razi2013; Wan et al., Reference Wan, Song, Diao, Li, Bao, Hu, Zhang and Zeng2014).
Our clinical study focused on the use of a laser zona thinning (laser-assisted hatching; LAH) method on day 3 cryopreserved embryos with the hypothesis that it may be a useful tool for improving live birth rates especially in advanced female age in frozen–thawed ICSI-ET cycles.
Materials and methods
Patient selection, controlled ovarian stimulation, oocyte recovery
Laser-assisted hatching procedure has been used for 11 years at our IVF Centre in the clinical work (Kanyo & Konc, Reference Kanyo and Konc2003). All patients (age: 21–43 years) received written information/coverage about the study and were asked to agree to the conditions of the study and to take part in it. They signed a written consent. Patients were allocated randomly into the LAH+ and LAH− groups based on the last number/digit of their registration number given to them by the administrator at the reception desk during their first visit to our centre. The embryologists were not informed about the deployment of the patients.
In total, 413 (203 with LAH+ and 210 with no LAH−) undertook thaw–transfer cycles within a 12-month period were analysed in the Infertility and IVF unit of a Medical Centre. All patients met the following criteria: (1) maximum three previous IVF-embryo transfer (IVF-ET); and (2) having reached ET process. Indications for IVF-ET were: male factor (40%), tubal factor (34%), endometriosis (15%) and unexplained infertility (11%). The reader is referred to our previous publications for stimulation protocols, oocyte recovery and ET techniques (Kanyo & Konc, Reference Kanyo and Konc2003; Konc et al., Reference Konc, Kanyo and Cseh2005, Reference Konc, Kanyo, Varga, Kriston and Cseh2010; Zeke et al., Reference Zeke, Konc, Kanyo, Kriston and Cseh2012).
On the day of embryo thawing and ET, the patients – according to the random allocation done during their first visit to the center when they signed up for the IVF program – were grouped into two groups: the LAH+ group and the LAH− group. The aim was to determine if LAH could increase the pregnancy rate in frozen ET cycles.
Fresh embryo culture
The G series sequential culture system (VitroLife, Gothenburg, Sweden) was used for in vitro culture of embryos. Embryo culture was performed as described previously (Konc et al., Reference Konc, Kanyo and Cseh2005, Reference Konc, Kanyo, Varga, Kriston and Cseh2010; Zeke et al., Reference Zeke, Konc, Kanyo, Kriston and Cseh2012).
Cryopreservation of embryos
Embryos were frozen on day 3 after in vitro fertilization with ICSI in phosphate-buffered saline solution (PBS) supplemented with 1.5 M 1,2-propanediol (PrOH), 25 mg/ml human serum albumin (HSA) and 0.1 M sucrose (S) (VitroLife, Gothenburg, Sweden). For freezing, a conventional slow freezing protocol was used with a PLANER III Kryo 10 cell freezer (Planer Products Ltd, Sunbury-on-Thames, UK). After seeding at −7°C, the embryos were slowly cooled (0.3°C/min) to −30°C, then the temperature was decreased rapidly (−10°C/min) to −80°C before plunging the embryos into liquid nitrogen (LN2).
Only good quality day 3 embryos were frozen. Good quality embryos were defined as those having regular blastomeres, <20% fragments and no multinucleated blastomeres and those containing at least seven cells on day 3 in both LAH and control groups.
Thawing protocol
During embryo thawing first air thaw was applied for 20 s followed by immersion into 30°C water bath for 20 s. After thawing, PrOH was removed from the cells in three steps with solutions containing PrOH in decreasing concentrations and 0.2 M S (VitroLife, Gothenburg, Sweden). After dilution out of the cryoprotectant, embryos were cultured in vitro for 1–1.5 h prior to final quality assessment and ET. Cryopreserved embryos were considered to have survived if >50% of the blastomere were intact. LAH was used just before ET on embryos in the LAH group (n = 276). In the control group, no LAH was used on embryos prior to ET. A maximum of 2–3 embryos was transferred per patient.
Laser-assisted hatching (LAH) of embryos
Embryo thawing was performed in the morning of ET, and LAH was carried out just before ET. LAH was performed only on embryos that survived following thawing (see before). Assisted hatching of human embryos was performed with a ‘non-contact’ 670 nm, 1.48 μm, infra-red diode laser (Fertilase™, MTM, Germany) (Table 1). A 1.48 μm continuous wave laser beam, which was collimated with a 1 mW visible 670 nanometer diode laser beam were fed into an inverted microscope through several mirrors and focused by a ×45 microscope objective. This led to a measured spot size of 1–3 μm in diameter. This spot was magnified and observed on an external monitor. The infra-red diode laser beam, focused through the microscope objective, is activated by using a foot pedal and causes a trench or a tunnel of the ZP. The drilling effect is due to a highly localized disruption of ZP glycoprotein matrix, which is stimulated by heat without causing ionization. The tangential laser irradiation results in a trench or tunnel-like hole (the diameter of the hole is 4.5–20 μm), at an optimal laser power setting of 47 mW.
Table 1 Patient characteristics and embryo transfer data in the laser and no-laser groups

The ZP was exposed for 10–15 ms to the laser beam. The diameter of the drilled holes varied between 5–10 μm depending on the irradiation time and the temperature of the surrounding medium. One or a maximum of two shots were performed. The embryos were transferred within 20 min (Kanyo & Konc, Reference Kanyo and Konc2003).
Endometrial preparation and transfer of frozen–thawed embryos
First, patients were downregulated starting in the mid-luteal phase of the preceding menstrual cycle. Subsequently, endometrial development was supported by administration of 17β-estradiol valerate (Estrofen®, tabl. 2 mg, Novo Nordisk) in increasing doses (2–6 mg/day) to mimic the natural cycle which was commenced orally on day 2 of the menstrual cycle and continued until endometrial thickness reached >8 mm. Once endometrial development was confirmed by ultrasound, progesterone was administered (600 mg/day; Utrogestan, Lab. Besins Internat S.A.) to support the luteal phase.
Embryo transfer was carried out on day 3 and prior to ET, LAH was performed on each embryo (MTG, Fertilase, Hamburg, Germany). Pregnancy was defined as a spontaneous rise in a β-hCG concentration at least 10 days post-transfer. Clinical pregnancy implied the presence of an intrauterine gestational sac and fetal heart beat on an ultrasound performed at 7 weeks of gestation (Zeke et al., Reference Zeke, Konc, Kanyo, Kriston and Cseh2012). In case of spontaneous abortion, the patient lost her biochemical or clinical pregnancy.
Statistical analysis
Data were analyzed by logistic regression using S-PLUS 2000. The outcome variable was the clinical pregnancy and the predictor was preparation of the embryos (LAH+ and LAH−) to ET. A comparison was also made between age, body mass index (BMI) and the number of transferred embryos and clinical pregnancy rate. Patients whose clinical pregnancy ended with a birth were considered as pregnant. Biochemical pregnancies were excluded from this group. Power calculations were also made and we reported a 95% confidence interval (95% CI) for the odds ratios (OR) associated with the factors included in the model.
Results
In the frame of this study, the results of 413 frozen–thawed ET cycle were evaluated. Out of the 413 ET cycles in 203, LAH was applied on the embryos just prior to transfer. In the control group, none of the transferred embryos was treated with LAH. The mean female age (at the time of the fresh ET), duration of infertility, mean number of previous failed ART cycles and, main BMI, number of biochemical or clinical pregnancies, number of spontaneous abortions were similar in the LAH+ and LAH− groups (Table 1).
Likewise, cryo-survival was also similar in the two groups (LAH+: 92.0% vs. LAH−: 91.4%). Comparing LAH+ and LAH− groups in the overall patient population, we could detect that LAH supports (33.3 % vs. 27.4%, respectively, P = 0.08; Fig. 1) the formation of clinical pregnancy. The statistical analyses, however, show only a trend but the results were not significantly different. When we compared the pregnancy rates of the LAH+ and LAH− groups based on the age of the patients, we found that in patients who were over 37 (≥38) years of age, LAH significantly increased the pregnancy rates (18.36% vs. 11.36%, respectively, P = 0.03) (Table 2). In patients under the age of 37, ‘only’ a non-significant increase (trend/tendency) in the pregnancy rate was detected in the LAH+ group (28.4% vs. 23.6%, respectively). In younger patients (<38 years), however, our data clearly show that patients’ age had a positive supportive effect on clinical pregnancy establishment, and younger patients got pregnant more easily (P = 0.003) compared with older patients.
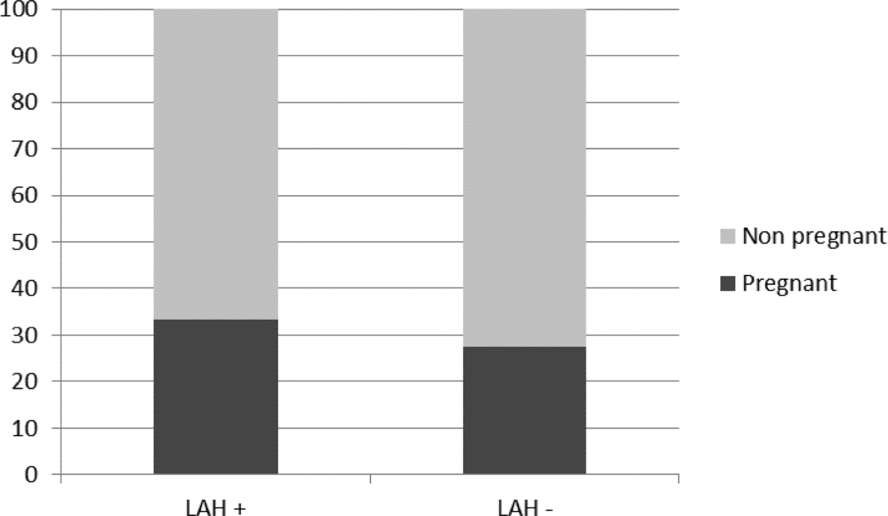
Figure 1 The overall pregnancy rates obtained in the LAH+ and LAH− groups.
Table 2 Patient characteristics and embryo transfer data in the laser and no-laser groups of patients with above 37 years of age

Our data also indicate that the number of transferred embryos has a strong positive influence on clinical pregnancy (two embryos: 10/49, 20%; three embryos: 8/29, 27%; four embryos: 1/3, 33%). But for patients above the age of 37, we were unable to prove statistically the supportive effect of the number of transferred embryos. Likewise, no effect of BMI on clinical pregnancy rate was observed. However, not only the clinical pregnancy rate, but the incidence of multiple pregnancies, was increased in the LAH+ group (5.8% vs. 3.3% for LAH+ and LAH−, respectively, P < 0.01).
Discussion
Prior to implantation, the blastocyst has to hatch from the ZP. The premise behind AH is based on the assumption that a modification of the human ZP might promote hatching and implantation of embryos that are otherwise unable to escape from the intact ZP (Cohen et al., Reference Cohen, Alikani, Trowbridge and Rosenwaks1992; Cohen, Reference Cohen, Elder and Cohen2007). The modification could be carried out either by its elimination, by drilling a hole, by thinning, or by altering its stability. The use of different AH procedures is based on the assumption that creating an artificial hole/opening or thinning the ZP might assist the hatching process of embryos.
AH supports in vivo hatching of the blastocyst and therefore may improve the efficacy of pregnancy rates. Assisted hatching was reported to increase the outcome of IVF-ET cycles in groups of patients with poor prognosis and/or poor embryonic morphology, with elevated FSH levels, with embryos having thicker ZP, who were older and with a history of multiple IVF failures. Thick ZP may be associated with advanced female age and poor embryo scores, and furthermore with cryopreservation (Cohen et al., Reference Cohen, Alikani, Trowbridge and Rosenwaks1992; Cohen, Reference Cohen, Elder and Cohen2007; Valojerdi et al., Reference Valojerdi, Eftekhari, Karimian and Ashtiani2008; Carney et al., Reference Carney, Das, Blake, Farquhar, Seif and Nelson2012; Wan et al., Reference Wan, Song, Diao, Li, Bao, Hu, Zhang and Zeng2014).
There is a sharp contradiction in the results and observations of different studies dealing with AH. Because of the discrepancy in the outcomes between the different studies, results obtained from them should be interpreted with caution (Ng et al., Reference Ng, Naveed, Lau, Yeung, Chan, Tang and Ho2005; Ge et al., Reference Ge, Zhou, Zhang and Lin2008; Valojerdi et al., Reference Valojerdi, Eftekhari, Karimian and Ashtiani2008; Feng et al., Reference Feng, Hershlag, Scholl and Cohen2009; Lan et al., Reference Lan, Huang, Lin, Kung and Chang2009; Carney et al., Reference Carney, Das, Blake, Farquhar, Seif and Nelson2012; Wan et al., Reference Wan, Song, Diao, Li, Bao, Hu, Zhang and Zeng2014).
Comparing the pregnancy rates of fresh IVF cycles with poor prognosis patients (prior IVF failures) after using different techniques of AH, no difference was found in the efficacy of these and all of the techniques (laser, chemical or microsurgical) increased pregnancy rates (Feng et al., Reference Feng, Hershlag, Scholl and Cohen2009). However, Baruffi et al. (Reference Baruffi, Mauri, Petersen, Ferreira, Coelho and Franco2000) in a prospective randomized study found no difference in pregnancy rates in a population aged <37 years and with no previous implantation failure (Baruffi et al., Reference Baruffi, Mauri, Petersen, Ferreira, Coelho and Franco2000). Moreover, Frydman et al. (Reference Frydman, Madoux, Hesters, Duvernoy, Feyereisen, Le Du, Tachdijian, Frydman and Fanchin2006) found that LAH did not improve the IVF-embryo transfer outcome in woman aged >37 years (Frydman et al., Reference Frydman, Madoux, Hesters, Duvernoy, Feyereisen, Le Du, Tachdijian, Frydman and Fanchin2006).
Cryopreservation may induce zona hardening similarly to in vitro culture and advanced female age (Larman et al., Reference Larman, Sheehan and Gardner2006). Assisted hatching may overcome this problem and increase implantation and pregnancy rates in frozen–thawed ET cycles. Similarly to the fresh IVF cycles, the results obtained after AH in frozen-ET cycles are very conflicting (Carney et al., Reference Carney, Das, Blake, Farquhar, Seif and Nelson2012).
Assisted hatching with chemical zona drilling in frozen–thawed cycles has been shown to increase pregnancy rates in retrospective studies (Cohen et al., Reference Cohen, Lindheim and Sauer1999). However, in other studies using similar techniques no advantage of the procedure could be detected in cryopreserved ICSI-ET cycles (Edirisinghe et al., Reference Edirisinghe, Ahnonkitpanit, Promviengchai, Suwajanakom, Pruksananonda, Chinpilas and Virutamasen1999). In a recently published randomized study, Ng et al. (Reference Ng, Naveed, Lau, Yeung, Chan, Tang and Ho2005) failed to show any beneficial effect of LAH on pregnancy rate following the transfer of cryopreserved embryos (Ng et al., Reference Ng, Naveed, Lau, Yeung, Chan, Tang and Ho2005). Primi et al. (Reference Primi, Senn, Montag, Van der Ven, Mandelbaum, Veiga, Barri and Germond2004) were also unable to show any benefit of AH in frozen–thawed ET cycles (Primi et al., Reference Primi, Senn, Montag, Van der Ven, Mandelbaum, Veiga, Barri and Germond2004). In the Ng et al. (Reference Ng, Naveed, Lau, Yeung, Chan, Tang and Ho2005) study, patients of advanced age also did not benefit from LAH, they were able to detect only a trend towards increased pregnancy rates (Ng et al., Reference Ng, Naveed, Lau, Yeung, Chan, Tang and Ho2005). Their observation is in contrast with ours, as we found that LAH increased significantly the pregnancy rate in patients over the age of 37. In younger patients, less than 37 years old, we could see only a trend of increased pregnancy rate in the LAH+ group. LAH improved the pregnancy rates in patients with frozen–thawed embryos, but they observed no effect in patients with advanced female age.
Our results obtained in frozen–thawed ICSI-ET cycles show that LAH increases pregnancy rates. The patient population was quite similar in the two groups, likewise the embryo characteristics and the applied cryopreservation and embryo culture procedures, thus precluding any bias that may have influenced our results. Similarly to our results, other studies also found that LAH significantly increased pregnancy rates in cryopreserved cycles with slowly frozen and vitrified embryos compared with fresh cycles (Ebner et al., Reference Ebner, Moser and Tews2005; Zhang et al., Reference Zhang, Yang, Lv, Min, Li and Bai2009).
The possible explanation of the less successful application of LAH could be that the size of the ZP thinning may have an effect on the results. Zhang et al. (Reference Zhang, Yang, Lv, Min, Li and Bai2009) compared the effect of the size of the ZP thinning (40 μm vs. 80 μm) made by the laser on the outcome of frozen–thawed ET cycles. They found that the size of the thinning by the laser may influence the implantation and pregnancy rates (they got increased implantation and pregnancy when 80 μm thinning was made) following frozen–thawed cleaved embryo transfer (Zhang et al., Reference Zhang, Yang, Lv, Min, Li and Bai2009). The data from Hiraoka et al.’s study (Reference Hiraoka, Hiraoka, Horiuchi, Kusuda, Okano, Kinutani and Kinutani2009) show that in vitrified-warmed embryo transfers the size of the ZP thinning area by LAH impacts the clinical pregnancy rate and that one-half ZP thinning significantly increases the results compared with one-quarter ZP thinning (Hiraoka et al., Reference Hiraoka, Hiraoka, Horiuchi, Kusuda, Okano, Kinutani and Kinutani2009).
Conclusion
In summary, the data from our study show that LAH may be useful for improving the pregnancy rates using frozen day 3 ET in recurrent pregnancy failure patients over 37 years. According to our results, LAH increased pregnancy rates in woman who were aged over 37 years and taking part in FET. In patients younger than 37 years old we could detect only a trend towards increased pregnancy rates in the LAH+ group compared with the LAH− group. The number of transferred embryos has a strong positive effect on pregnancy formation, but only in patients younger than 37 years of age. In people over 37 years old this positive effect of embryo number on pregnancy could not be detected. The incidence of multiple pregnancies was increased in the LAH+ group. Considering our data and to overcome the negative effect of zona hardening, which is very typical in older recurrent pregnancy failure woman, plus the fact that cryopreservation makes the situation more difficult, we suggest that LAH could be performed on frozen–thawed embryos as a routine strategy in FET cycles before they are transferred.







