During recent years milk proteins have been recognised as a valuable source of bioactive peptides, demonstrating various health benefits in humans. The content of these proteins may vary between different species(Reference Miranda, Mahé and Leroux1, Reference Uniacke-Lowe, Huppertz and Fox2). Many of the derived peptides display antibacterial activity against a broad spectrum of bacterial strains, both Gram-positive and Gram-negative(Reference Tomita, Takase and Bellamy3–Reference Lopez-Exposito, Recio and Bosze5). These milk peptides are mainly characterised by low molecular weight (MW), an increased number of ionic groups and an exposure of hydrophobic groups(Reference Panyam and Kilara6). All the naturally occurring whey proteins, such as β-lactoglobulin (β-LG), α-lactalbumin (α-LA), immunoglobulins, lactoperoxidase, lysozyme (LZ) and lactoferrin (LF), including the glycomacropeptides in cheese whey, have been reported to be the source of bioactive peptides when digested enzymically(Reference Meisel and Schlimme7–Reference Pihlanto and Korhonen9).
It is well known that LF, LZ, lactoperoxidase and immunoglobulins possess properties that inhibit bacterial growth, as part of the natural host defence system in humans protecting against a great number of pathogenic micro-organisms(Reference van Hooijdonk, Kussendrager and Steijns10–Reference Korhonen, Marnilla and Gill12). Fragments of β-LG prepared with commercial enzymes such as alcalase, pepsin or trypsin, produce peptides that inhibit several types of bacteria, both Gram-positive and Gram-negative(Reference Meisel and Schlimme7, Reference El-Zahar, Sitohy and Choiset13). Other studies have shown that digestion of α-LA with pepsin, trypsin or chymosin release antimicrobial peptides(Reference Pellegrini, Thomas and Bramaz14). In addition, glycomacropeptide has received much attention due to its ability to attach to enterotoxins from various bacteria; for example kappacin, a monophosphorylated sequence, has been reported to possess antibacterial activity against Streptococcus mutans and Escherichia coli (Reference Malkoski, Dashper and O'Brien-Simpson8).
The most studied antibacterial components in milk and whey are probably LF and LZ. Both generate peptides during enzymic hydrolysis and the peptides possess strong inhibitory effects against various bacteria. Mine et al. (Reference Mine, Ma and Lauriau15) identified two antibacterial peptides produced from LZ, by pepsin and subsequent tryptic digestion, which demonstrated strong inhibitory effects against Staphylococcus aureus and E. coli. During the last decade, research on peptides derived from LF has received increased attention since the derivatives strongly inhibit both Gram-positive and Gram-negative bacteria(Reference Wakabayashi, Takase and Tomita16–Reference Haque and Chand18). Several of these antimicrobial peptides have been sequenced and synthesised, including bovine lactoferricin f(17–41) and lactoferrampin f(268–284)(Reference Yamauchi, Tomita and Giehl19, Reference van der Kraan, Groenink and Nazmi20). These peptides showed a broad antibacterial effect against strains of E. coli, Bacillus subtilis, Staphylococcus aureus, Salmonella enterica and Listeria monocytogenes (Reference Murdock and Matthews21). On the other hand, it has also been reported that LF can increase the growth of bacteria such as probiotic strains of Lactobacillus (Reference Sherman, Bennett and Hwang22).
Although most of the research has been performed with bovine milk, similar results have also been observed for human, ovine, murine, equine, donkey and caprine milk. One of these peptides, lactoferricin C, has been identified as caprine lactoferrin f(14–42)(Reference Recio, Slangen and Visser23). This peptide showed strong antimicrobial activity against various types of bacteria(Reference Kimura, Nam and Ohkouchi24, Reference Recio and Visser25). In most previous studies commercial proteolytic enzymes from animal or plant origin were used(Reference Tomita, Takase and Bellamy3, Reference Haque and Chand18, Reference Tomita, Bellamy and Takase26). The questions therefore arise whether these peptides are released during human gastrointestinal digestion and in what quantity are they generated. Finally, the physiological relevance in humans remains to be proved.
Only a few human studies have been performed that could confirm the many in vitro studies using proteolytic enzymes. Human ingestion of an LF solution (1·5 %) showed that only 20 % of holo- and 38 % apo-LF was digested in the stomach(Reference Troost, Steijns and Saris27). Another digestion study with milk and yoghurt as test meals showed that very few fragments derived from whey proteins were released during digestion(Reference Chabance, Marteau and Rambaud28). We have previously shown that digestion of whey proteins is very dependent on the gastric pH. At pH 2, a significantly higher degradation of whey proteins was observed by human gastrointestinal enzymes as compared with at pH 4(Reference Eriksen, Halvor and Jensen29). Since pH in the human stomach seems to vary with age and buffering capacity of the diet, the peptides generated may also vary between individuals, leading to highly variable physiological effects. It has been shown previously that β-LG, α-LA and LZ in bovine and caprine milk are very resistant to digestion with human gastrointestinal enzymes(Reference Aabøe Inglingstad, Devold and Eriksen30) and peptides generated from these proteins will probably be present in a rather low concentrations. Digestion of milk proteins from other species may be different, as β-LG from equine milk(Reference Aabøe Inglingstad, Devold and Eriksen30) was rapidly degraded by human gastrointestinal juices.
Human gastrointestinal enzymes are a complex mixture of proteases, amylases and lipases that exist in different isoforms in combination with inhibitors, bile salts, bilirubin and other minor components that may all influence protein degradation(Reference Scheele, Bartelt and Bieger31, Reference Dunn32). In a study performed with β-LG the degradation profile was very different after the addition of bile salts(Reference Gass, Vora and Hofmann33). Consequently, purified commercial enzymes from animal or plant origin and human digestion juices seem to generate different peptides from caprine whey(Reference Eriksen, Halvor and Jensen29, Reference Almaas, Holm and Langsrud34). Peptides available to the intestinal brush-border surface after digestion may be structurally different and display different physiological effects.
The objective of the present study was, first, to examine whether antibacterial peptides were produced from caprine whey after human gastrointestinal digestion and, second, to compare the peptides obtained with previously identified peptides using purified non-human enzymes.
Materials and methods
Whey protein concentrate from caprine milk
Caprine milk was collected from the university farm, and caprine whey protein concentrate (WPCG) with about 81 % (w/v) protein was produced by rennet precipitation and ultra-filtration at the university pilot plant(Reference Almaas, Holm and Langsrud34). WPCG is denoted as sample A in the antibacterial screening results.
Aspiration and human gastrointestinal enzymes
Human proteolytic enzymes were obtained according to Almaas et al. (Reference Almaas, Holm and Langsrud34) and Holm et al. (Reference Holm, Hanssen and Krogdahl35). The present study was carried out to follow up and extend our previous studies on in vitro digestion of caprine milk and whey. The gastric and duodenal juices were obtained from the same individual as previously described (healthy male, no medical treatment) consisting of pepsin and total proteolytic activities that are close to the mean value observed in eighteen individuals (men and women; EK Ulleberg, I Comi, H Holm, EB Heggset, M Jacobsen and GE Vegarud, unpublished results)(Reference Eriksen, Halvor and Jensen29). In brief, aspiration was performed by a three-lumen tube that enabled simultaneous instillation of saline in the duodenum and aspiration of gastric and duodenal juice. Saline (100 ml/h) was instilled close to the papilla of Vater and duodenal juice aspirated some 18 cm distally. The juice was immediately cooled down and frozen at − 20°C. Aspirates were collected several times during a period of 6 months. Before further use the aspirated samples of gastric and duodenal juice were pooled into two separate batches to avoid variations in enzyme activity. The aspirate containing the gastric juice was characterised by pH and pepsin activity (U/ml) and the duodenal juice by pH and total proteolytic activitity (U/ml). Pepsin activity in the human gastric juice was assayed with Hb as the substrate(Reference Sánchez-Chiang, Cisternas and Ponce36). Total proteolytic activity in the human duodenal juice was assayed with casein as the substrate(Reference Krogdahl and Holm37). A unit of enzyme activity (1 U) is defined as the amount of enzyme that produces an absorbance reading of optical density (OD) 1·0 at 280 nm in 20 min at 37°C. More than three parallels of the enzyme assays were used.
In vitro model digestion
A modified in vitro digestibility assay (AOAC official method 982.30)(Reference Rasco and Nielsen38) was performed in two steps, using human gastric juice and human duodenal juice according to Almaas et al. (Reference Almaas, Holm and Langsrud34).
A protein sample of 10 ml 5 % (w/v) WPCG (81 % protein) was acidified to pH 2·5 with 2 m-HCl, and incubated with 50 μl (0·4 U) human gastric juice for 30 min at 37°C. pH was adjusted to pH 7–8 with 1 m-NaOH, and 400 μl (13 U) human duodenal juice was added during continuous stirring for 30 min at 37°C. Samples were redrawn during the digestion, put on ice, frozen and then freeze-dried. The hydrolysate generated from the first step of digestion with human gastric juice was denoted sample B, while the hydrolysate obtained from the second step of degradation with both human gastric and duodenal juices was denoted sample C. The digestion was performed more than three times.
Separation of protein fractions by size membrane filtration
Fraction B from human gastric juice and fraction C from human duodenal juice digestion were separated in various subfractions using membranes with cut-offs at 5 and 8 kDa.
Fractions B and C were both prepared as 5 % solutions (50 g/l). The samples were filtered tangentially through a membrane with a cut-off of 8 kDa (Pellicon 2; Millipore, Billerica, MA, USA). Fraction C < 8 kDa was further separated by size filtration on a membrane with a cut-off of 5 kDa (Mini Ultra Omega SC membrane; Pall Corp., Port Washington, NY, USA). The filtrations were performed with a Masterflex pump (Millipore) and tubings (Masterflex AG, Gelsenkirchen, Germany), with pressure at 0·5 bar (7·5 psi (pounds per square inch)). The subfractions were kept on ice, and three to four washings through the membranes were carried out. The subfractions were freeze-dried after filtration. An overview of the different protein fractions is given in Table 1.
Table 1 Protein fractions of caprine whey protein concentrate (WPCG), prepared by digestion with human gastric juice (HGJ) for 30 min and human duodenal juice (HDJ) for 30 min at 37°C, and further separated into subfractions by size membrane filtration

MW, molecular weight.
Desalting and concentration of the fractions
Freeze-dried hydrolysates and subfractions were dissolved in 0·1 % (v/v) formic acid (FA). The samples were desalted and concentrated using self-made columns consisting of C18 column material (3 M Empore C18 extraction discs; 3M Bioanalytical Technologies, St Paul, MN, USA) inserted into Eppendorf GELoader micropipette tips (Hamburg, Germany). The peptides were eluted using 2 μl 70 % acetonitrile–0·1 % FA (v/v).
Identification of peptides by nano-LC–MS
Eluted peptides were diluted in 10 μl 1 % (v/v) FA before they were loaded onto a nanoAcquityTM Ultra Performance LC (Waters Corp., Milford, MA, USA), containing a 3 μm Symmetry® C18 Trap column (180 μm × 22 mm) (Waters Corp.) in front of a 3 μm AtlantisTM C18 analytical column (100 μm × 100 mm) (Waters Corp.). Peptides were separated with a gradient of 5–90 % (v/v) acetonitrile–0·1 % (v/v) FA, with a flow of 0·4 μl/min eluted to a Q-TOF Ultima Global mass spectrometer (Micromass, Waters Corp.) and subjected to data-dependent tandem MS analysis. Peak lists were generated by ProteinLynx Global server software (version 2.1; Waters Corp.), and the resulting pkl files were searched against the National Center for Biotechnology Information (NCBI) non-redundant protein sequence databases using the MASCOT search engine (http://www.matrixscience.com). Peptide mass tolerance used in the search was 100 parts per million; fragment mass tolerance was 0·1 Da. Data were acquired over a mass/charge range of 300–1500 Da, detecting peptides with two or three charges. Then twenty-two peptides were selected and synthesised by GenScript (GenScript USA Inc., Piscataway, NJ, USA) with 85 % purity (see Table 2) based on peptide sequences from β-LG, β-casein and κ-casein glycomacropeptide (Figs 1–3) identified by the LC–MS.
Table 2 Percentage inhibition of the synthesised single peptide sequences (0·1 mg/ml), and their protein precursors, κ-casein (κ-CN), β-casein (β-CN), β-lactoglobulin (β-LG), bovine glycomacropeptide (GMP) and bovine lactoferrin (LF) on Escherichia coli K12, Bacillus cereus RT INF01 and Listeria monocytogenes after 10 h growth*

* All samples were run in triplicate.

Fig. 1 Full-length amino acid sequence of β-lactoglobulin and identified peptides (forty-three framed) generated by digestion with human gastrointestinal enzymes from human gastric juice (30 min) and human duodenal juice (30 min) at 37°C.

Fig. 2 Full-length amino acid sequence of β-casein and identified peptides (twenty-five framed) generated by the digestion with human gastrointestinal enzymes from human gastric juice (30 min) and human duodenal juice (30 min) at 37°C.

Fig. 3 Full-length amino acid sequence of κ-casein glycomacropeptide (106–169) and identified peptides (twenty-three framed) generated by the digestion with human gastrointestinal enzymes from human gastric juice (30 min) and human duodenal juice (30 min) at 37°C.
Analysis of identified peptides
Of the identified peptides, nineteen were chosen to include all residues detected with minimal overlap. These peptides were analysed using Clustal 2.0.12 multiple sequence alignment (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The alignment was analysed using the multiple sequence editor(Reference Waterhouse, Procter and Martin39) (http://www.jalview.org/). Default settings were used for both programs.
Bacterial strains and culture conditions
E. coli K12, Staphylococcus aureus American Type Culture Collection (ATCC) 25 923 and Bacillus cereus RT INF01 were all obtained from the department stock collection at the Norwegian University of Life Sciences (UMB;Ås, Norway). Listeria monocytogenes, a culture of four undefined strains and Lactobacillus rhamnosus GG (LGG®; ATCC 53 103) were donated by Tine BA (Oslo, Norway). The cheese starter culture CHR CH-N01 was obtained from Christian Hansen Laboratory AS (Hørsholm, Denmark). This culture is a mixture of Lactococcus lactis subsp. lactis (1–5 %), Lactococcus lactis subsp. cremoris (70–80 %), Lactococcus lactis subsp. diacetylactis (10–20 %) and Leuconostoc mesenteroides subsp. cremoris (5–18 %).
E. coli K12 and Listeria monocytogenes were cultured in brain heart infusion (BHI) broth (Oxoid; 37 g/l) at pH 7·4 and 37°C. Staphylococcus aureus ATCC25923 and the mixed strain starter culture CH-N01 were grown at 37°C in M17-broth (42·5 g/l, pH 7·2; Merck).
Bacillus cereus RT INF01 and LGG® (ATCC 53 103) were cultured in de Man–Rogosa–Sharpe (MRS) broth (52·2 g/l, pH 5·7; Merck) at 37°C. Active growing cultures (1 %) were used for inoculation in the growth experiments.
Assay of antibacterial activity
Freeze-dried samples of WPCG and hydrolysates (fractions A, B and C) were solubilised in water and added to growing bacteria cultures. The final protein and hydrolysate concentrations varied from 0·3 to 1·2 %(Reference Almaas, Holm and Langsrud34). These concentrations were selected since 0·6 % is the concentration of whey proteins in milk(Reference Fox and McSweeney40). The synthesised peptide (GensScript) concentration used was 0·1 mg/ml. Bacterial growth was measured by OD at 660 or 600 nm. The experiments were repeated three times for each sample.
The number of viable cells (colony-forming units) was counted on agar plates for strains of E. coli, B. cereus and Listeria monocytogenes. E. coli and Listeria monocytogenes were grown on BHI–agar plates (Merck; 37 g/l) at pH 7·4 and 37°C, and B. cereus on MRS–agar plates (Merck; 22·5 g/l) at pH 7·0 at 37°C. All plates, three parallels of each dilution – 10− 7, 10− 8 and 10− 9 – were incubated for 48 h and then counted. Each experiment was repeated three times.
Calculations and statistics
Growth inhibition was expressed as optical density (OD600 nm) after 10 h in comparison with the control:
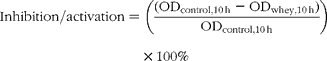
where ODcontrol,10 h is the OD for the control bacterial curve after 10 h, and ODwhey,10 h is the OD for the bacterial curve with the addition of the digested whey or peptide in the growth media after 10 h.
A t test (two-sample, assuming unequal variances) was run to compare the different growth-curves based on data obtained after 10 h. Each experiment was repeated three times with at least three parallels, and the differences were considered significant when P < 0·05. All the OD600 nm measurements (recorded every 30 min) were calculated for standard deviation. The graphs are presented as mean values and standard deviations after 10 h. The rest of the standard deviation bars have been omitted for clarity in the figures.
Results
Identified peptides generated from caprine whey proteins after in vitro gastrointestinal digestion
Digestion of caprine WPCG (fraction A) was carried out in two steps; first, with human gastric juice at pH 2·5 and, subsequently, with human duodenal juice at pH 7–8, resulting in hydrolysates called fractions B and C, respectively (Table 1). The hydrolysate (fraction C) was separated into subfractions according to the molecular size; high MW>8 kDa, medium MW 5–8 kDa and low MW < 5 kDa. Peptides generated from the two-step digestion were identified by LC–MS analysis. Five peptides were identified after human gastric juice digestion, having MW 8264, 9091 and 9918 Da and of 1845 and 2190 Da. After total digestion with human gastric and duodenal juices, 106 peptides were identified originating from β-LG, β-casein derivatives of γ-caseins, κ-casein glycomacropeptide and LF. Peptides, forty-three in all, were derived from β-LG representing peptide fragments of the whole sequence of the molecule ranging from 856·4 to 3436·8 Da (Fig. 1). Fragments from β-casein, twenty-five peptides, were located mainly from the middle part of the protein (Fig. 2). The twenty-three peptides generated from the κ-casein glycomacropeptide, as a component in renneted cheese whey, were derived mainly from the N-terminal side of molecule (Fig. 3). In addition, fifteen peptides derived from LF were located in the middle of the molecule (Fig. 4). Lactoferricin f(17–42) or lactoferrampin f(268–284) were not detected, nor were any peptides originating from α-LA.

Fig. 4 Full length amino acid sequence of lactoferrin (1–791) and identified peptides (fifteen in black) generated by the digestion with human gastrointestinal enzymes from human gastric juice (30 min) and human duodenal juice (30 min) at 37°C.
The results of multiple sequence alignment of nineteen peptide sequences from β-LG, β-casein and κ-casein glycomacropeptide are given in Fig. 5. The results showed a consensus sequence, LTPVPELK, including two prolines (P) with a valine (V) in between and neighbouring the bulky hydrophobic leucine (L). Such proline-rich sequences have been described as antimicrobial peptides(Reference Pripp, Isaksson and Stepaniak41).

Fig. 5 Clustal multiple sequence alignment of nineteen peptides. Peptides no. 1–7 are derived from β-lactoglobulin, no. 8–16 from β-casein and no. 17–19 from κ-casein glycomacropeptide. The consensus sequense, LTPVPELK, is shown with leucine (L), proline (P) and valine (L).
Antibacterial effect of hydrolysates and peptides generated after digestion
The antibacterial effect of WPCG and the generated hydrolysates was tested in three different concentrations (0·3, 0·6 and 1·2 %). The results showed similar trends for all concentrations. Data from only 0·6 % are presented in the following part. From the growth curves of the various bacteria the growth rate and percentage inhibition were calculated (Table 3). The results obtained varied highly between the bacteria. E. coli K12 showed significant growth inhibition by the addition of the hydrolysate generated by gastric juice (fraction B) and an increased inhibitory effect after both human gastric and duodenal juice digestion (fraction C), as shown in Table 3. Although fraction C strongly inhibited growth (27 %), this effect seemed to be exhibited by components in the subfraction with MW>8 kDa, since subfractions with MW < 8 kDa and < 5 kDa showed no inhibition. From the distribution of the 106 identified peptides in the molecules, twenty-two peptides were selected for synthesis and antibacterial testing. All the peptides showed a relatively moderate inhibition of E. coli K12 (Table 2). The peptide fragment f(191–205) derived from β-casein showed the highest antibacterial effect, with approximately 14 % inhibition. However, all peptides were less active than the hydrolysates obtained after human gastric juice and human duodenal juice digestion (fraction C), showing 27 % inhibition (Table 3). This fraction was bacteriocidal since a loss of viable E. coli K12 cells (measured as colony-forming units) was observed (data not shown). A clear reduction in growth rate (ΔOD600 nm/h) was also shown by the same fraction C (Table 3).
Table 3 Percentage growth inhibition of Escherichia coli, Bacillus cereus and Listeria monocytogenes after 10 h (optical density (OD) at 600 nm) comparing control culture without added protein with protein fractions and subfractions

WPCG, caprine whey protein concentrate; MW, molecular weight.
* P < 0·05, ** P < 0·005.
† Growth rate was calculated in the logarithmic growth phase between 2 and 4 h after inoculum.
After gastric digestion of whey (fraction B) no growth inhibition of Staphylococcus aureus, B. subtilis, Listeria monocytogenes, LGG and the cheese starter culture CHR CH-01 was observed. However, subsequent duodenal digestion (fraction C) resulted in strong activity against Listeria monocytogenes and B. cereus, with 38 and 44 % inhibition, respectively, after 10 h growth (Table 3). For both strains it seemed to be the subfraction of high MW (MW>8 kDa) that was most active (38 and 41 % inhibition). The lower-MW subfractions (MW < 8 kDa and MW < 5 kDa) showed less antibacterial effect, except on the cheese starter culture CHR CH-01. Only four of the twenty-two synthesised peptides showed a slight antibacterial effect (5–7 % inhibition) against B. cereus and Listeria monocytogenes. These four peptides were derived from κ-casein glycomacropeptide. All the other peptides derived from β-LG and β-casein had no inhibitory effect. The high-MW subfraction (MW>8 kDa) of the digested whey showed high antibacterial effect; therefore, two proteins reported as antibacterial, bovine κ-casein glycomacropeptide and bovine LF (BLF), were tested. No inhibition was shown by glycomacropeptide while bovine LF showed only moderate (8 %) inhibition (Table 2).
Discussion
Antibacterial peptides from milk and whey proteins have been reported during the last 20 years with clear inhibitory effects on various strains of E. coli, Listeria monocytogenes, B. cereus and other micro-organisms(Reference Tomita, Takase and Bellamy3, Reference Malkoski, Dashper and O'Brien-Simpson8, Reference Haque and Chand18, Reference Recio and Visser25). However, all of these bioactive peptides have been obtained through hydrolysis with commercial enzymes of animal or plant origin. Purified non-human enzymes degrade milk proteins more efficiently to shorter peptides(Reference Eriksen, Halvor and Jensen29, Reference Almaas, Cases and Devold42, Reference Almaas, Berner and Holm43). Addition of bile salt also seems to change the protein degradation of β-LG(Reference Gass, Vora and Hofmann33). The presence of other components apart from proteases seems to be important in the overall protein degradation. Human gastric and duodenal juices contain a complex mixture of proteases, amylases, lipases, inhibitors, bile salts, bilirubin and other minor components that may have an important role in the total human gastrointestinal digestion.
Proteins digested with non-human and human enzymes seem to generate different peptides both with regard to sequence and length(Reference Eriksen, Halvor and Jensen29). When comparing the 106 identified peptides from human enzymes with previously identified peptides from purified commercial enzymes, only two or three peptides matched. One of these peptides derived from β-LG f(92–100) has been reported earlier in both bovine and caprine species(Reference Haque and Chand18). Another peptide, called casecidin 15, having the sequence f(191–205) derived from caprine β-casein has been previously reported in bovine colostrum(Reference Birkemo, O'Sullivan and Ross44). However, the reported casecidin 17 f(191–207) from κ-casein was not identified in the present study. These are surprising results, considering the many identical amino acid sequences in the caprine and bovine milk proteins.
A relatively high amount of proline seemed to be present in the nineteen peptide sequences shown by multiple sequence alignment analysis. A clustering sequence, LTPVPELK, containing two prolines with a valine and two hydrophobic leucines could constitute a possible common motif that plays a role in the proteolytic attack by human enzymes. This is in accordance with reports that proline restricts proteolytic processing(Reference Jörnwall and Persson45). Short proline-rich sequences together with hydrophobic residues such as leucine and phenylalanine have also been described as antimicrobial peptides(Reference Shinnar, Butler and Park46).
Another observation in conflict with previously published reports was the absence of peptides identified from LF. No lactoferricin, LFcinC f(14–42), or lactoferrampin, LFampinC f(268–284), was identified in the present study even though these peptides have been reported with animal proteolytic enzymes and have also been identified in the gastrointestinal tract of mice(Reference Bellamy, Takase and Wakabayashi47, Reference Kuwata, Yip and Yamauchi48). However, the in vivo studies by Troost et al. (Reference Troost, Steijns and Saris27) and Chabance et al. (Reference Chabance, Marteau and Rambaud28) showed that most of the LF was intact after gastric digestion (30 min) and only a few peptides were identified from whey proteins in milk after 30 min, 2 h and 4 h of ingestion.
Concerning the high potent antibacterial effect reported in the literature by purified peptides from milk proteins(Reference Murdock and Matthews21, Reference Recio and Visser25, Reference Birkemo, O'Sullivan and Ross44), a relatively low effect of peptides derived from β-LG, β-casein and κ-casein glycomacropeptide on E. coli K12, B. cereus and Listeria monocytogenes was shown in the present study. The hydrolysate obtained after gastrointestinal digestion of whey had a much stronger antibacterial effect than the single peptides. This might be due to either a low concentration of peptides used or that the hydrolysate contained a complex mixture of high- and low-MW proteins and peptides that may act in a synergistic manner. Surprisingly, neither the peptides nor the digested whey had any antimicrobial effect on the probiotic strain LGG; it seemed rather to be activated by the hydrolysate. This may play a role in fermented milk products such as milk and yoghurts that are on the market today.
It should be realised that the amount of peptides released from whey protein with gastrointestinal enzymes is relatively low, since 65–70 % of β-LG and 90–98 % of α-LA are still intact after human gastric and duodenal juice digestion. Peptides from β-LG only were identified and no peptides from α-LA(Reference Eriksen, Halvor and Jensen29, Reference Aabøe Inglingstad, Devold and Eriksen30). These results seems to be in agreement with in vivo studies of Chabance et al. (Reference Chabance, Marteau and Rambaud28) showing that only a few peptides from whey proteins were detected in the duodenum after human ingestion of milk or yoghurt. Questions arise why proteins such as β-LG and α-LA are more or less resistant to degradation and whether they and other polypeptides are degraded further in the jejunum or by intracellular proteases.
In conclusion, the present study showed that human gastrointestinal enzymes generate few peptides from caprine whey after gastric digestion compared with duodenal digestion. Identification of the peptides in the hydrolysates was different from previously reported peptides using purified non-human enzymes. Strong antibacterial effects were observed on E. coli, B. cereus and Listeria monocytogenes. Pure peptides were less inhibitory compared with the fractionated whey hydrolysates. No effect was shown on the probiotic strain LGG. Host-protective activity of whey as a digestion product is an interesting dietary aspect that might be significant for public health.
Acknowledgements
The present study was approved by the Norwegian Ethical Board.
The present study was supported by the the Indo-Norwegian programme, the Norwegian University of Life Sciences (UMB) Food and Health Strategic programme and the Trust Hospital of Østfold.
The contributors to the present study were H. A. in her PhD work on digestion studies, PhD student E. E. on peptide identification, C. S. and technical assistant I. C. in all bacteriology assays, R. F. and E. J. in protein chemistry and proteomics, H. H. in human aspiration, M. J. in medical gastroenterology, T. L. in biochemistry and G. E. V. as project leader.
There are no conflicts of interest to declare.












