Introduction
Weeds are among the most economically important pests of agronomic crops worldwide and are a major constraint to optimum crop production (Jhala et al. Reference Jhala, Knezevic, Ganie, Singh, Chauhan and Mahajan2014). Herbicides have become integral to weed control in agronomic crop production systems particularly after the commercialization and widespread adoption of herbicide-resistant (HR) crops (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012). The use of herbicides has provided economical and selective weed control in major agronomic crops in developed countries for many years (Duke Reference Duke2015), although widespread and repeated use of herbicides has resulted in the evolution of HR weeds (Beckie Reference Beckie2006; Kniss Reference Kniss2018). As of 2021, there are at least 502 unique cases of HR weeds worldwide in 263 weed species, including 152 broadleaf and 111 grass weeds (Heap Reference Heap2021). Grass weeds are usually more difficult to identify compared with broadleaf weeds, particularly at the seedling stage due to similar appearance and emergence as a single leaf (Sarangi and Jhala Reference Sarangi and Jhala2017). Because growers rarely proactively manage HR grass weeds, their widespread occurrence and interference in agronomic crop production fields present a challenge (Beckie Reference Beckie2007). The grass weed species, rigid ryegrass, has evolved resistance to at least 14 herbicides with different sites of action (SOAs) in more than 21 countries (Suzukawa et al. Reference Suzukawa, Bobadilla, Mallory-Smith and Brunharo2021).
Pollen-mediated gene flow (PMGF) is the change in the frequency of alleles in a population due to the movement of pollen via wind, insect pollinators, or other means (Ganie and Jhala Reference Ganie and Jhala2017) leading to gene introgression in the population (Jhala et al. Reference Jhala, Norsworthy, Ganie, Sosnoskie, Beckie, Mallory-Smith, Liu, Wei, Wang and Stoltenberg2021). While seed-mediated gene flow is very common in most weed species (Beckie et al. Reference Beckie, Blackshaw, Hall and Johnson2016), gene flow can also occur through the transfer of vegetative propagules in certain weed species such as creeping bentgrass (Mallory-Smith and Zapiola Reference Mallory-Smith and Zapiola2008). PMGF occurs, at least to some extent, in most flowering plants, including grass weeds, and usually creates genetic diversity through the exchange of alleles within and between populations (Jhala et al. Reference Jhala, Norsworthy, Ganie, Sosnoskie, Beckie, Mallory-Smith, Liu, Wei, Wang and Stoltenberg2021). The intensity of gene exchange between populations via PMGF primarily depends on the plant breeding system (Darmency Reference Darmency and Brown1996), the pollen migration dynamics of the donor and recipient populations (Busi et al. Reference Busi, Michel, Powles and Délye2011; Délye et al. Reference Délye, Clement, Pernin, Chauvel and Le Corre2010), distance between populations (Busi et al. Reference Busi, Yu, Barrett-Lennard and Powles2008), and plant density of the source population (Baker and Preston Reference Baker and Preston2006; Rognli et al. Reference Rognli, Nilsson and Nurminiemi2000). If the pollen source population is large enough and has a high frequency of herbicide resistance alleles, PMGF can cause effective migration of genes to distant population with a low plant density (Mallory-Smith et al. Reference Mallory-Smith, Hall and Burgos2015), as evidenced with isolated, herbicide-susceptible rigid ryegrass (Busi et al. Reference Busi, Yu, Barrett-Lennard and Powles2008). Genetic variation among individuals within a population is required for adaptive evolution by natural selection. The abundant genetic variation detected within a species may explain why resistance to many different herbicide SOAs has evolved so rapidly in certain weed species such as Italian ryegrass.
The evolution of HR weeds can be explained by analyzing population genetic principles to understand the continuous trade-off between the intensity of herbicide selection, the in situ mutation rate endowing herbicide resistance alleles, and the pleiotropic fitness effects of resistance-endowing traits (Diggle and Neve Reference Diggle, Neve, Powles and Shaner2001; Gressel Reference Gressel1978; Maxwell et al. Reference Maxwell, Roush and Radosevich1990). These factors drive the rate of evolution of resistance or increase the frequency of resistance alleles in a population (Jhala et al. Reference Jhala, Norsworthy, Ganie, Sosnoskie, Beckie, Mallory-Smith, Liu, Wei, Wang and Stoltenberg2021). The movement of herbicide resistance alleles within or between fields is the gene flow that impacts the spread and distribution of HR weeds (Jasieniuk et al. Reference Jasieniuk, BruleBabel and Morrison1996; Neve et al. Reference Neve, Busi, Renton and Vila-Aiub2014). Empirical studies have determined that PMGF in cross-pollinated species can be high even at long distances depending on the species (Levin and Kerster Reference Levin and Kerster1974) compared with self-pollinated species (Jhala et al. Reference Jhala, Norsworthy, Ganie, Sosnoskie, Beckie, Mallory-Smith, Liu, Wei, Wang and Stoltenberg2021). This phenomenon has been documented to occur over considerable distances at the landscape level at relatively high frequency in some wind-pollinated grasses (Watrud et al. Reference Watrud, Lee, Fairbrother, Burdick, Reichman, Bollman, Storm, King and Van de Water2004) such as creeping bentgrass (Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2017) and has generally been modeled by a negative exponential decay distribution (Levin and Kerster Reference Levin and Kerster1974; Pfender et al. Reference Pfender, Graw, Bradley, Carney and Maxwell2007).
PMGF and the spread of HR weeds into new agroecosystems is a concern because it introduces new HR alleles into weeds and makes them difficult to control with herbicides (Jhala et al. Reference Jhala, Bhatt, Keith and Hall2011; Jhala and Hall Reference Jhala, Hall and Price2013). Beckie et al. (Reference Beckie, Busi, Bagavathiannan and Martin2019) reported that PMGF from HR crops has been extensively studied; however, PMGF from HR weeds is understudied. Jhala et al. (Reference Jhala, Norsworthy, Ganie, Sosnoskie, Beckie, Mallory-Smith, Liu, Wei, Wang and Stoltenberg2021) reviewed PMGF from economically important HR broadleaf weeds to susceptible weeds; however, a comprehensive review of the scientific literature on PMGF from HR grass weeds has not been collated. The objective of this article was to review reproductive biology, HR biotypes, and the potential for herbicide resistance allele transfer via pollen from economically important and widely distributed HR grass weeds, including barnyardgrass, creeping bentgrass, Italian ryegrass, johnsongrass, rigid ryegrass, and wild oats (Avena fatua L. and Avena sterilis L.).
Barnyardgrass
Barnyardgrass is the world’s principal grass weed in rice (Mitich Reference Mitich1990). It is among the most troublesome weeds in crops grown in rotation with rice or crops grown in areas where moist soil or seasonal flooding is common. Barnyardgrass is native to Europe and Asia and is found in almost all the agricultural regions of the world (Mitich Reference Mitich1990). It is a C4 semi-terrestrial summer annual grass weed with high fecundity (Bagavathiannan et al. Reference Bagavathiannan, Norsworthy, Smith and Neve2012; Bosnic and Swanton Reference Bosnic and Swanton1997; Norris et al. Reference Norris, Elmore, Rejmánek and Akey2001), rapid turnover of its soil seedbank (Bagavathiannan et al. Reference Bagavathiannan, Norsworthy, Smith and Burgos2011), and tremendous genetic diversity (Altop and Mennan Reference Altop and Mennan2011). In Asian countries where hand weeding of transplanted rice is a common practice, barnyardgrass has evolved to morphologically mimic rice, making its removal from the crop difficult (Barrett Reference Barrett1983). Research continues to show high morphological and genetic variability between individual barnyardgrass genotypes within geographic locations, which aids the adaptive nature of this weed (Altop and Mennan Reference Altop and Mennan2011; Rutledge et al. Reference Rutledge, Talbert and Sneller2000).
Barnyardgrass Reproductive Biology
Barnyardgrass is a self-compatible and an autogamous grass weed species (Maun and Barrett Reference Maun and Barrett1986) that exhibits indeterminate flowering and production of panicles with reproduction mainly through selfing (Figure 1; Table 1), although outcrossing can occur, especially when plants are in close proximity (Bagavathiannan and Norsworthy Reference Bagavathiannan and Norsworthy2014). There is no known published literature on the viability of barnyardgrass pollen, and gene flow is mostly associated with wind events.

Figure 1. Ongoing pollination of a barnyardgrass inflorescence (photo credit: Jason Norsworthy).
Table 1. Economically important herbicide-resistant grass weed species reviewed in this study, their chromosome numbers, and their likelihood of pollen-mediated gene flow.

a Likelihood of pollen-mediated gene flow (PMGF) was estimated based on reproductive biology and scientific literature.
b Chromosome numbers reported for barnyardgrass vary from 2n = 36, 42, 48, 54, and 72 (Aoki et al. Reference Aoki and Yamaguchi2008).
c Creeping bentgrass is an allotetraploid species; johnsongrass is a tetraploid species.
d Wild oats are hexaploid species.
Herbicide Resistance in Barnyardgrass
Barnyardgrass and the closely related junglerice [Echinochloa colona (L.) Link] have evolved resistance to 13 herbicide SOAs with resistance to as many as seven SOAs in some populations, making management of this weed challenging in many cropping systems (Rouse et al. Reference Rouse, Roma-Burgos, Norsworthy, Tseng, Starkey and Scott2018). It is one of the most HR-prone weed species, second to rigid ryegrass (Heap Reference Heap2021). Based on results from simulation modeling, simultaneous application of two effective herbicides has a greater likelihood of mitigating resistance in barnyardgrass compared with the exhaustion of one effective herbicide followed by the use of another (Bagavathiannan et al. Reference Bagavathiannan, Norsworthy, Smith and Neve2014).
Florpyrauxifen-benzyl, a synthetic auxinic herbicide, is the most recent novel herbicide commercialized for use in rice for barnyardgrass control. Prior to the commercialization of this herbicide, results from herbicide screening programs indicated that florpyrauxifen-benzyl was effective for controlling quinclorac-resistant barnyardgrass and for its resistance to other SOA herbicides (Miller et al. Reference Miller, Norsworthy and Scott2018). Recent findings, however, show that florpyrauxifen-resistant barnyardgrass is present in rice production fields, with the resistance mechanism appearing to be based on non-target sites (Hwang et al. Reference Hwang, Norsworthy, Torralva, Priess, Barber and Butts2022). Limited herbicide options in crops such as rice, the occurrence of multiple herbicide resistance involving non-target site resistance mechanisms in barnyardgrass, and the lack of discovery of novel herbicide target sites make the sustainability of existing herbicide programs questionable at the current rate of herbicide loss due to resistance.
PMGF from HR Barnyardgrass
As with other weeds, gene flow in barnyardgrass can occur via seed or pollen movement. In field studies conducted in Arkansas, the likelihood of PMGF from barnyardgrass was examined using a quinclorac-resistant population as a pollen source (Bagavathiannan and Norsworthy Reference Bagavathiannan and Norsworthy2014). Wind was found to be the main factor aiding the movement of resistance alleles via pollen, with very low PMGF detected at 50 m (0.01%), the farthest distance studied. Based on these findings, the authors speculated that seed movement would most likely contribute to the landscape-level spread of herbicide resistance in barnyardgrass through rainfall events, birds, and human activities. Likewise, Rutledge et al. (Reference Rutledge, Talbert and Sneller2000) suggested that the spread of herbicide resistance in barnyardgrass is primarily a result of seed dispersal following the selection of independent mutation events.
Creeping Bentgrass
Creeping bentgrass is a perennial, cool season (C3) turf grass often used on golf courses, especially putting greens, due to its ability to withstand repeated, low mowing and foot traffic (Ferguson Reference Ferguson, Hanson and Juska1969). More than 80% of creeping bentgrass seed sold globally is produced in Oregon. In 2019, 1.3 million kg with a market value of US$8.73 million were produced on approximately 2,000 ha (ODA 2020). Many of the species in the Agrostis genus, including creeping bentgrass, are weedy or invasive in managed and unmanaged sites (Ahrens et al. Reference Ahrens, Chung, Meyer and Auer2011; Hart et al. Reference Hart, Belanger, McCullough and Rotter2009; Reichman et al. Reference Reichman, Watrud, Lee, Burdick, Bollman, Storm, King and Mallory-Smith2006). The weed risk potential of creeping bentgrass is classified as high (USDA-APHIS 2014; Table 1). Creeping bentgrass is widespread globally and is reported in all states in the continental United States (MacBryde Reference MacBryde2006). It is a known grass weed in roadsides, ditches, irrigation canals, turf, and pasture (Figure 2). In the United States, the genus includes both native and nonnative species (MacBryde Reference MacBryde2006).

Figure 2. Glyphosate-resistant creeping bentgrass established on an irrigation canal in 2016 near Ontario, Oregon (photo credit: Maria Zapiola).
Creeping Bentgrass Reproductive Biology
Creeping bentgrass is an outcrossing, self-incompatible, wind-pollinated species that reproduces via seed and stolons (Bradshaw Reference Bradshaw1958; Kik et al. Reference Kik, van Andel and Joenje1990). Creeping bentgrass produces flowers on a panicle and flowers over an extended period of time. Pollen grains of creeping bentgrass are approximately 35.4 µm in diameter and weigh on average 52 ng (Pfender et al. Reference Pfender, Graw, Bradley, Carney and Maxwell2007). The seeds are very small, with about 13,500 seeds g−1. The seeds are easily transported via human activity and natural vectors such as wind, water, and animals. Panicles in irrigation canals moved an average of 19 m min−1 (Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2010; Figure 2). Creeping bentgrass seeds soaked in water for 17 wk (the limits of the study) at 4 and 20 C maintained germinability (Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2010).
As a perennial species, once a plant is established, it can contribute PMGF over multiple years, and genes can also disperse via seeds and stolons. Seeds of glyphosate-resistant creeping bentgrass lines survived more than 4 yr of burial in Oregon (Hancock et al. Reference Hancock, Park and Mallory-Smith2015). In greenhouse and laboratory studies, plants were established from sections of stolons with one node buried at 15 cm, and stolons remained viable after 5 d of desiccation at 20 C or after being submerged in water up to 2 m at 4 C (Dysart and Mallory-Smith unpublished data).
The Agrostis genus contains about 200 species, many of which are sexually A. ×murbeckii compatible with creeping bentgrass. Naturally occurring intraspecific, interspecific, and intergeneric (most commonly with Polypogon ssp.) hybrids have been identified (MacBryde Reference MacBryde2006). Some of the hybrids are sufficiently common to have been given a scientific name; for example, the hybrid A. ×murbeckii Fouill. is a cross between creeping bentgrass and A. capillaris. Agrostis ×murbeckii is sterile but reproduces via stolons and is reported to be more competitive than either parent (Bradshaw Reference Bradshaw1958). Many of the hybrids are fertile; even if sterile, the plants will likely persist because the parent species are perennial and reproduce vegetatively.
Herbicide Resistance in Creeping Bentgrass
Amitrole-resistant creeping bentgrass was identified in 1986 in Belgium (Bulke and Eelen Reference Bulke and Eelen2000). The HR biotype was found in a pear orchard where long-term studies had been conducted. This is the only report of evolved resistance in this species. Although never commercialized, genetically engineered glyphosate-resistant and glufosinate-resistant creeping bentgrass have been grown. Glyphosate-resistant creeping bentgrass was produced by Scotts Company and Monsanto using the CP4 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene used in other glyphosate-resistant crops (Zapiola et al. Reference Zapiola, Campbell, Butler and Mallory-Smith2008). Glyphosate-resistant creeping bentgrass was grown for seed production in field sites in Oregon in 2002 and Idaho in 2002 to 2005 (Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2017).
Glufosinate-resistant creeping bentgrass was produced by several entities, often the result of using the bar gene as a marker in plant transformation (Asano et al. Reference Asano, Ito, Fukami, Sugiura and Fujiie1998; Hartman et al Reference Hartman, Lee, Day and Turner1994; Lee et al. Reference Lee, Hartman, Laramore, Tumer and Day1995, Reference Lee, Kim, Kim, Lee, Kim and Lee2010; Wang et al. Reference Wang, Browning, Ruemmele, Chanlee, Kausch and Jackson2003). Lee et al. (Reference Lee, Kim, Kim, Lee, Kim and Lee2010) produced creeping bentgrass with resistance to both herbicides. The authors are unaware of reports of feral glufosinate-resistant creeping bentgrass occurring in the environment as there is with glyphosate-resistant creeping bentgrass (Zapiola et al. Reference Zapiola, Campbell, Butler and Mallory-Smith2008); however, it would not be surprising to find such cases due to research being conducted in field experiments, and because there would be no expectation that the glufosinate-resistant plants would respond differently than glyphosate-resistant plants (Belanger et al. Reference Belanger, Meagher, Day, Plumley and Meyer2003a, Reference Belanger, Plumley, Day and Meyer2003b; Lee et al. Reference Lee, Hartman, Laramore, Tumer and Day1995; Wipff and Fricker Reference Wipff and Fricker2000).
PMGF from HR Creeping Bentgrass
PMGF from both glyphosate- and glufosinate-resistant creeping bentgrass has been evaluated in greenhouse and field studies in Oregon. Greenhouse studies used the glufosinate resistance gene as a marker to determine the frequency of interspecific crosses between creeping bentgrass and four Agrostis species, velvet, dryland, and colonial bentgrasses, and redtop (Belanger et al. Reference Belanger, Plumley, Day and Meyer2003b). Glufosinate-resistant hybrids were recovered from all four species. The number of resistant hybrids ranged from 8% to 22% depending on the maternal parent. Under field conditions, the hybrids produced in the greenhouse study were fertile and could backcross with the parental species.
Field studies have included experimental designs using either glyphosate- or glufosinate-resistant plants as pollen sources and compatible species as pollen receptor plants spaced at known distances from the pollen source (Belanger et al. Reference Belanger, Meagher, Day, Plumley and Meyer2003a; Christoffer Reference Christoffer2003; Wipff and Fricker Reference Wipff and Fricker2000). Several studies were conducted over relatively short distances, ranging from 15 m (Belanger et al. Reference Belanger, Meagher, Day, Plumley and Meyer2003a) to 350 m (Christoffer Reference Christoffer2003). Christoffer (Reference Christoffer2003) used glyphosate-resistant creeping bentgrass as the pollen parent and 15 other species as pollen receptors, 12 Agrostis species and three Polypogon species. (Table 2). Hybrids were produced on all tested species with the exception of A. nebulosi and Apera interrupta. No interspecific or intergeneric hybrids were found beyond 50 m, whereas intraspecific hybrids were recovered at 354 m, which was the limit of the study. Belanger et al. (Reference Belanger, Meagher, Day, Plumley and Meyer2003a) reported crosses between glufosinate-resistant creeping bentgrass and A. capillaris and A. castellana. Wipff and Fricker (Reference Wipff and Fricker2001) reported crosses between glufosinate-resistant creeping bentgrass and five Agrostis species (Table 2). Wipff and Fricker (Reference Wipff and Fricker2001) measured intraspecific gene flow from glufosinate-resistant creeping bentgrass to conventional bentgrass. They recovered resistant plants at 300 m, which was the limit of the study. These studies confirm the potential for herbicide resistance allele movement via pollen. In all cases, gene flow was confirmed at the farthest distance measured in the study. Wipff and Fricker (Reference Wipff and Fricker2000) used an exponential decay model to predict gene flow at a level of 0.02%, which estimated pollen movement of approximately 1,300 m.
Table 2. Agrostis species reported to produce herbicide-resistant hybrids either in situ or in research trials.

a Many of these species have multiple synonyms for their scientific and common names.
Two large-scale studies were conducted in Oregon where glyphosate-resistant creeping bentgrass had been planted for seed production in Jefferson County in an area designated by the Oregon State Department of Agriculture. In 2002, eight fields totaling 162 ha were planted with glyphosate-resistant creeping bentgrass within the 4,500 ha area. One study evaluated gene flow outside of the control area and the other evaluated gene flow within the control area (Watrud et al. Reference Watrud, Lee, Fairbrother, Burdick, Reichman, Bollman, Storm, King and Van de Water2004; Zapiola et al. Reference Zapiola, Campbell, Butler and Mallory-Smith2008; Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2012, Reference Zapiola and Mallory-Smith2017).
For the study outside of the control area, pollen receptor plants were used but were placed at much greater distances than in other studies, with glyphosate-resistant creeping bentgrass seed production fields serving as the pollen sources (Watrud et al. Reference Watrud, Lee, Fairbrother, Burdick, Reichman, Bollman, Storm, King and Van de Water2004). Seeds recovered from the receptor plants and in situ feral creeping bentgrass and redtop plants were tested for the presence of the CP4 EPSPS gene. Resistant seedlings were found on receptor plants and in situ creeping bentgrass plants at 21 and 8 km distance, respectively. Resistant seedlings were produced on redtop plants at a maximum distance of 14 km. In a follow-up study, in situ glyphosate-resistant plants were identified up to 3.8 km from the perimeter of the control area in non-agricultural sites (Reichman et al. Reference Reichman, Watrud, Lee, Burdick, Bollman, Storm, King and Mallory-Smith2006). The authors suggested that these plants could have resulted from both pollen and seed movement and provided evidence that creeping bentgrass plants would be established outside of cultivation. The distance that pollen moved in this study is an underestimation of the actual distance because the plants were placed based on the control area perimeter rather than an individual field.
In a second study, the PMGF within and around the seed production control area was assessed and documented over a 4-yr period (Zapiola et al. Reference Zapiola, Campbell, Butler and Mallory-Smith2008). In 2006, 3 yr after the fields were taken out of production, 62% of the 585 creeping bentgrass tested in situ (established on irrigation ditches, canals, and roadsides) were glyphosate-resistant. The most distant resistant plants were found 4.6 km from the nearest seed production field. It was not possible to determine in all cases whether gene movement was due to pollen, seed, or stolons. Glyphosate-resistant hybrid seed of creeping bentgrass, A. gigantea, and P. monspelienis were produced on plants in situ (Zapiola et al. Reference Zapiola, Cronn and Mallory-Smith2010; Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2012, Reference Zapiola and Mallory-Smith2017). Hybrids between creeping bentgrass and A. gigantea produced both rhizomes and stolons, indicating a potential reproductive advantage over either parent (Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2017). An intergeneric hybrid between glyphosate-resistant creeping bentgrass and the annual P. monspelienis (×Agropogon littoralis) was identified in the study (Zapiola and Mallory-Smith Reference Zapiola and Mallory-Smith2012, Reference Zapiola and Mallory-Smith2017; Figure 3). Although previously reported to be male sterile, the hybrid produced viable seed. In addition, the resulting hybrid was perennial and produced stolons, traits that P. monspelienis does not have, could increase gene flow. In 2016, in situ glyphosate-resistant hybrid plants of creeping bentgrass by A. gigantea (Jefferson County OR) and ×Agropogon littoralis (Malheur County OR) were identified (Mallory-Smith and Zapiola unpublished data). The plasticity in the Agrostis genus and the number of potential crosses that can occur make identifying hybrids in the field challenging if not impossible; therefore, molecular markers are needed to determine parentage (Zapiola et al. Reference Zapiola, Cronn and Mallory-Smith2010). This difficulty in identifying hybrids may allow the gene to remain in the environment without detection.

Figure 3. (left to right) Spikes of glyphosate-resistant creeping bentgrass, intergeneric hybrid between glyphosate-resistant creeping bentgrass and annual rabbitsfoot, and annual rabbitsfoot (photo credit: Maria Zapiola).
Italian Ryegrass
Italian ryegrass is a highly competitive weed in cereal fields, vineyards, orchards, and natural grasslands in areas worldwide with temperate climate. The species is believed to be a native to the Mediterranean region of southern Europe, northwest Africa, and southwest Asia (Beddows Reference Beddows1973; Hubbard Reference Hubbard1968) but is now distributed in temperate regions of the majority of continents (Clayton et al. Reference Clayton, Vorontsova, Harman and Williamson2021). Similar to rigid ryegrass, it was intentionally introduced and continues to be cultivated for pasture forage, turf, and erosion control in many regions of the world, where it escaped cultivation to become a major agricultural weed (CABI 2021). According to Lacefield et al. (Reference Lacefield, Collins, Henning, Phillips, Rasnake, Spitaleri, Girgson and Turner2003), Italian ryegrass was first cultivated in northern Italy, followed by cultivation in France, Switzerland, and England. It was introduced into the United States in the early colonial migration and quickly became an important forage grass, especially in the South. Italian ryegrass is a major weed in cereal fields of the southeastern and south-central United States and in cereal fields, nut and fruit orchards, and vineyards in the Pacific Northwest. Italian ryegrass is a C3 bunchgrass with a summer annual, winter annual, or biennial life cycle (Beddows Reference Beddows1973) depending on the climatic conditions of the geographical region it inhabits. High intra- and interspecific diversity has been reported in this species (Maity et al. Reference Maity, Singh, Bastos Martins, Jose Ferreira, Smith and Bagavathiannan2021), allowing for high adaptive potential. It is especially invasive where ground cover is disturbed or discontinuous and requires fertile, well-drained soils, and moderate temperature and moisture conditions for vigorous growth and reproduction (Beddows Reference Beddows1973; CABI 2021).
Italian Ryegrass Reproductive Biology
Italian ryegrass is hermaphrodite and reproduces solely by sexual reproduction and seeds. Inflorescences consist of spikes 10 to 30 cm long that are comprised of spikelets 8 to 25 mm long, each with 5 to 20 laterally flattened fertile florets and smaller sterile florets at the apex (Beddows Reference Beddows1973; CABI 2021; Clayton et al. Reference Clayton, Vorontsova, Harman and Williamson2021). Spikelets alternate with one another along the rachis, which is recessed opposite each spikelet, and breaks up at maturity by disarticulating below each fertile floret. Each fertile floret has three yellow, occasionally tinged red, anthers and two feathery stigmas that protrude slightly before or at the same time as the anthers when the floret opens (Beddows Reference Beddows1973). Each floret produces one caryopsis (single-seeded fruit). Pollen is dispersed by wind.
Italian ryegrass is self-incompatible and outcrosses at extremely high levels (>99%; Fearon et al. Reference Fearon, Hayward and Lawrence1983). Self-incompatibility is determined by two multi-allelic gene loci (S and Z) with gametophytic control of the incompatibility phenotype (Fearon et al. Reference Fearon, Hayward and Lawrence1983). The incompatibility reaction takes place on the stigmatic surface. Incompatible pollen produces only short pollen tubes, whereas compatible pollen produces long tubes. Self-incompatibility, an extremely high outcrossing rate, and wind-mediated pollen dispersal results in abundant genetic variation within populations and low genetic differentiation among populations, as has been observed in Italian ryegrass in California (Karn and Jasieniuk Reference Karn and Jasieniuk2017). Genetic variation among individuals within a population is required for adaptive evolution by natural selection. The abundant genetic variation detected within Italian ryegrass populations may explain why resistance to many different herbicide SOAs has evolved so rapidly in this species.
Herbicide Resistance in Italian Ryegrass
Italian ryegrass populations have evolved resistance to herbicides with at least nine different SOAs (Ghanizadeh et al. Reference Ghanizadeh, Harrington and James2015; Heap Reference Heap2021), including acetyl CoA carboxylase (ACCase) inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Bravin et al. Reference Bravin, Zanin and Preston2001; Kaundun Reference Kaundun2010; Mahmood et al. Reference Mahmood, Mathiassen, Kristensen and Kudsk2016; Nandula et al. Reference Nandula, Giacomini, Lawrence, Molina and Bond2020; Rauch et al. Reference Rauch, Thill, Gersdorf and Price2010; Stranger and Appleby Reference Stranger and Appleby1989), acetolactate synthase (ALS) inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Délye et al. Reference Délye, Boucansaud, Pernin and Le Corre2009; Mahmood et al. Reference Mahmood, Mathiassen, Kristensen and Kudsk2016; Rauch et al. Reference Rauch, Thill, Gersdorf and Price2010; Shimono et al. Reference Shimono, Shimono, Oguma, Konuma and Tominaga2015), carotenoid biosynthesis inhibitors (Ghanizadeh et al. Reference Ghanizadeh, Harrington and James2015), EPSPS inhibitor (glyphosate; Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Dickson et al. Reference Dickson, Scott, Burgos, Salas and Smith2011; Jasieniuk et al. Reference Jasieniuk, Ahmad, Sherwood, Firestone, Perez-Jones, Lanini, Mallory-Smith and Stednick2008; Niinomi et al. Reference Niinomi, Ikeda, Yamashita, Ishida, Asai, Shimono, Tominaga and Sawada2013; Perez and Kogan Reference Perez and Kogan2003; Perez-Jones et al. Reference Perez-Jones, Park, Colquhoun, Mallory-Smith and Shaner2005; Preston et al. Reference Preston, Wakelin, Dolman, Bostamam and Boutsalis2009; Salas et al. Reference Salas, Dayan, Pan, Watson, Dickson, Scott and Burgos2012), glutamine synthase inhibitors (Avila-Garcia et al. Reference Avila-Garcia and Mallory-Smith2011, Reference Avila-Garcia, Sanchez-Olguin, Hulting and Mallory-Smith2012; Fernández et al. Reference Fernández, Alcántara, Osuna, Vila-Aiub and De Prado2017; Ghanizadeh et al. Reference Ghanizadeh, Harrington and James2015; Karn et al. Reference Karn, Beffa and Jasieniuk2018; Kurata et al. Reference Kurata, Ichihara, Ishida, Shimono and Tominaga2017), protoporphyrinogen oxidase (PPO) inhibitors (Fernández et al. Reference Fernández, Alcántara, Osuna, Vila-Aiub and De Prado2017), photosystem I inhibitors (Brunharo and Hanson Reference Brunharo and Hanson2018; Tehranchian et al. Reference Tehranchian, Nandula, Jugulam, Putta and Jasieniuk2018), photosystem II (PS II) inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Liu et al. Reference Liu, Hulting and Mallory-Smith2014), and very long chain fatty acid (VLCFA) inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Liu et al. Reference Liu, Hulting and Mallory-Smith2014, Reference Liu, Hulting and Mallory-Smith2016; Rauch et al. Reference Rauch, Thill, Gersdorf and Price2010). Resistance to ACCase inhibitors and the EPSPS-inhibiting herbicide glyphosate are the most frequently reported types of resistance in Italian ryegrass (Heap Reference Heap2021).
Multiple resistance to two, three, four, and five herbicide SOAs is becoming increasingly common in Italian ryegrass populations. Instances of multiple resistance to two SOAs include resistance to ACCase and ALS inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Chandi et al. Reference Chandi, York, Jordan and Beam2011; Kuk et al. Reference Kuk, Burgos and Talbert2000, Reference Kuk, Burgos and Scott2008; Rauch et al. Reference Rauch, Thill, Gersdorf and Price2010; Singh et al. Reference Singh, Maity, Abugho, Swart, Drake and Bagavathiannan2020), ACCase and EPSPS inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Dickson et al. Reference Dickson, Scott, Burgos, Salas and Smith2011), ALS and EPSPS inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Henckes et al. Reference Henckes, Cechin, Schmitz, Piasecki, Vargas and Agostinetto2019), and EPSPS and glutamine synthetase inhibitors (Avila-Garcia and Mallory-Smith Reference Avila-Garcia and Mallory-Smith2011; Fernández et al. Reference Fernández, Alcántara, Osuna, Vila-Aiub and De Prado2017; Karn et al. Reference Karn, Beffa and Jasieniuk2018; Kurata et al. Reference Kurata, Ichihara, Ishida, Shimono and Tominaga2017). Italian ryegrass populations with resistance to three herbicide SOAs have been confirmed (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Brunharo and Hanson Reference Brunharo and Hanson2018; Ghanizadeh et al. Reference Ghanizadeh, Harrington and James2015; Liu et al. Reference Liu, Hulting and Mallory-Smith2014, Reference Liu, Hulting and Mallory-Smith2016; Rauch et al. Reference Rauch, Thill, Gersdorf and Price2010; Singh et al. Reference Singh, Maity, Abugho, Swart, Drake and Bagavathiannan2020; Vázquez-García et al. Reference Vázquez-García, Alcántara-de la Cruz, Palma-Bautista, Rojano-Delgado, Cruz-Hipólito, Torra, Barro and De Prado2020a; Tehranchian et al. Reference Tehranchian, Nandula, Jugulam, Putta and Jasieniuk2018). Italian ryegrass populations with resistance to four herbicide SOAs include resistance to ACCase, ALS, PS II, and VLCFA inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Liu et al. Reference Liu, Hulting and Mallory-Smith2016); ACCase, ALS, EPSPS, and PS II inhibitors (Tehranchian et al. Reference Tehranchian, Nandula, Matzrafi and Jasieniuk2019); and ALS, EPSPS, PS II, and VLCFA inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021). Recently, 15 of 75 Italian ryegrass populations surveyed in the Willamette Valley of Oregon were confirmed with resistance to five herbicide SOAs, i.e., ACCase, ALS, EPSPS, PS II, and VLCFA inhibitors (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021).
PMGF from HR Italian Ryegrass
The high genetic diversity admixture and low differentiation detected between California populations (Karn and Jasieniuk Reference Karn and Jasieniuk2017) strongly suggest the spread of herbicide resistance alleles through PMGF; however, experimental studies of PMGF from HR Italian ryegrass are lacking. In contrast, several studies have been conducted for rigid and perennial ryegrass. Given the morphological and reproductive similarities, close genetic relatedness, and inter-fertility of the three species, the results of these studies are likely to be directly applicable to Italian ryegrass. Yanniccari et al. (Reference Yanniccari, Istilart, Giménez and Castro2018) studied PMGF in perennial ryegrass from glyphosate-resistant to glyphosate-susceptible plants under field conditions in Argentina using a donor-receptor design, and PMGF of glyphosate resistance reached susceptible trap plants up to 35 m downwind from the source of pollen. Susceptible receptor plants grown at 15 and 25 m from the source of glyphosate resistance exhibited a 4-fold and 2-fold increase, respectively, in the frequency of glyphosate-resistant offspring, whereas no glyphosate-resistant plants were detected in the progeny of susceptible receptor plants located >35 m from donor resistant plants.
Interspecific Hybridization in Lolium Species
Italian ryegrass readily hybridizes with perennial ryegrass and rigid ryegrass to produce hybrids where the species co-occur (Charmet et al. Reference Charmet, Balfourier and Chatard1996; Dinelli et al. Reference Dinelli, Bonetti, Lucchese, Catizone, Bravin and Zanin2002, Reference Dinelli, Bonetti, Marotti, Minelli and Catizone2004; Kloot Reference Kloot1983; Terrell Reference Terrell1966). All three species are diploid with 2n = 14 chromosomes (Terrell Reference Terrell1966) (Table 1), have high rates of outcrossing due to self-incompatibility (Fearon et al. Reference Fearon, Hayward and Lawrence1983), are highly variable morphologically (Bravin et al. Reference Bravin, Zanin and Preston2001; Terrell 1968; Vasek and Ferguson 1963), and have the same basic karyotype (Bulinska-Radomska and Lester 1985; Malik and Thomas Reference Malik and Thomas1966).
Intergeneric hybridizations between Italian ryegrass and Festuca species have also been detected in nature (Dinelli et al. Reference Dinelli, Bonetti, Lucchese, Catizone, Bravin and Zanin2002, Reference Dinelli, Bonetti, Marotti, Minelli and Catizone2004; Zwierzykowski Reference Zwierzykowski1996). Interestingly, a significantly higher amount of Festuca DNA was detected in two diclofop-resistant Italian ryegrass biotypes than in a susceptible accession in Italy, suggesting the possibility of resistance gene flow from Festuca into Italian ryegrass via hybridization (Dinelli et al. Reference Dinelli, Bonetti, Marotti, Minelli and Catizone2004). However, based on the homology between chromosomes of certain Lolium and Festuca species, some authors have suggested that the two genera may share a monophyletic origin (Bulinska-Radomska and Lester Reference Bulinska-Radomska and Lester1988). Thus, the resistance trait shared by the interfertile genera (Lolium and Festuca) could have been inherited from a common ancestor. The evolution of resistance to diclofop in Italian ryegrass as a consequence of Festuca species gene introgression was also suggested by Martinez-Ghersa et al. (Reference Martinez-Ghersa, Ghersa, Vila-Aiub, Satorre and Radosevich1997), but scientific support for the hypothesis is lacking.
Johnsongrass
Native to the Mediterranean, johnsongrass (2n = 4x = 40) has a wide geographical range between 55oN and 45oS, and is distributed in more than 53 countries across Asia, Africa, Australia, Europe, North America, and South America (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977). Molecular genetic investigations suggest that S. halepense is a hybrid of S. bicolor (2n = 2x = 20) and S. propinquum (2n = 2x = 20; Paterson et al. Reference Paterson, Schertz, Lin, Liu and Chang1995). Chromosome doubling in S. propinquum has been suggested as a possible origin of S. halepense (Defelice Reference Defelice2006). Johnsongrass was introduced into the United States in the late 1700s (McWhorter Reference McWhorter1971), and molecular evidence suggests two geographically distinct introduction events (Ohadi et al. Reference Ohadi, Hodnett, Rooney and Bagavathiannan2017; Uzay Sezen et al. Reference Uzay Sezen, Barney, Atwater, Pederson, Pederson, Chandler, Cox, Cox, Dotray, Kopec, Smith, Schroeder, Wright, Jiao, Kong, Goff, Auckland, Rainville, Pierce, Lemke, Compton, Phillips, Kerr, Mettler and Paterson2016).
Being a perennial, rhizomatous, invasive, C4 grass species, johnsongrass is a troublesome weed in summer crops in the southern United States (McWhorter Reference McWhorter1989). Johnsongrass is a particularly problematic weed in sorghum fields (Figure 4) due to its genetic similarities to sorghum and, until recently, the lack of selective herbicide options. The weediness of johnsongrass is attributed to its rapid vegetative growth and biomass accumulation, vigorous rhizome production, high fecundity, seed dormancy, and seedbank longevity, among other factors (McWhorter Reference McWhorter1989; Warwick and Black Reference Warwick and Black1983). Plants show a high degree of intra-population variability for adaptive traits, which might contribute to its range expansion into northern environments (Burt and Wedderspoon Reference Burt and Wedderspoon1971). The high relative growth rate and adaptation to water stress suggests increased frequency of johnsongrass occurrence under future climate change scenarios, especially under rainfed conditions (Leguizamon and Acciaresi Reference Leguizamon and Acciaresi2014).

Figure 4. Infestation of johnsongrass in a grain sorghum production field in southeast Texas (photo credit: Muthukumar Bagavathiannan).
Johnsongrass Reproductive Biology
Johnsongrass reproduces via both seeds and underground rhizomes. The flowering stems (culms) grow up to 2.5 m tall and about 2.0 cm diameter (Warwick and Black Reference Warwick and Black1983). It produces large, erect, open, spreading panicles that can extend up to 50 cm in length. The spikelets appear in pairs, except at the end of each branch where they occur in triplets. Seeds usually have awns (1.0 to 1.6 cm long), which are generally shed at maturity. The mature grains are oblong to ovate, reddish brown to shiny black and remain enclosed in the glumes even after complete maturity (Warwick and Black Reference Warwick and Black1983).
A single johnsongrass plant can produce about 28,000 seeds (Horowitz Reference Horowitz1973), which easily shatter upon maturity, leading to wide dispersal. In addition to seeds, a substantial number of rhizomes (up to about 90 m total length) can be produced in a growing season (McWhorter and Jordan Reference McWhorter and Jordan1976), which can account for up to 70% of the plant’s total dry mass (Oyer et al. Reference Oyer, Gries and Rogers1959). Each rhizome can grow up to about 2 m in length and several cm in diameter, with internodes covered with brown sheaths (Figure 5). The cut pieces of rhizomes can regenerate into individual plants, thus contributing to their spread. Liu et al. (Reference Liu, Scursoni, Moreno, Zelaya, Sol Munoz and Kaundun2019) developed an individual-based model of johnsongrass that incorporated seed and rhizome propagation and revealed the important role of rhizome production in driving population dynamics.

Figure 5. Vigorous rhizome production by johnsongrass in a field study conducted at College Station, Texas (photo credit: Muthukumar Bagavathiannan).
Herbicide Resistance in Johnsongrass
As of 2020, 29 unique cases of herbicide resistance in johnsongrass have been reported in 12 countries worldwide, including Argentina, Australia, Chile, Greece, Hungary, Israel, Italy, Mexico, Serbia, Spain, the United States, and Venezuela (Heap Reference Heap2021). Resistance has been reported for the EPSPS inhibitor glyphosate, ACCase inhibitors, ALS inhibitors, and the microtubule inhibitors. A case of multiple-herbicide resistance in johnsongrass was documented in Argentina with resistance to both glyphosate and haloxyfop-methyl. In the United States, HR johnsongrass biotypes have been reported in Arkansas, Indiana, Kentucky, Louisiana, Mississippi, Tennessee, Texas, Virginia, and West Virginia. In a multi-state johnsongrass survey conducted across northern Kansas, northwestern Missouri, and southern Nebraska, Werle et al. (Reference Werle, Jhala, Yerka, Dille and Lindquist2016) found that 8% of the populations (5 out of 59) were resistant to imazethapyr and 5% (3 out of 59) were resistant to nicosulfuron. Barrentine et al. (Reference Barrentine, Snipes and Smeda1992) reported johnsongrass resistance to fluazifop-P in two field populations in Mississippi. Smeda et al. (Reference Smeda, Snipes and Barrentine1997) further studied these populations and found they also exhibited resistance to quizalofop-P, along with reduced susceptibility to sethoxydim. Johnson et al. (Reference Johnson, Norsworthy and Scott2014) screened for the presence of herbicide resistance in 141 johnsongrass accessions collected during 2008–2010 across 14 counties in Arkansas and found resistance in two accessions for glyphosate or imazethapyr. Riar et al. (Reference Riar, Norsworthy, Johnson, Scott and Bagavathiannan2011) confirmed glyphosate resistance in a johnsongrass population collected from a field near West Memphis, AR, that had been under continuous soybean production for at least 6 yr with frequent use of glyphosate. This resistant population was 5-fold to 7-fold less sensitive to glyphosate compared to the susceptible population.
In Europe, Vázquez-García et al. (Reference Vázquez-García, Palma-Bautista, Rojano-Delgado, De Prado and Menendez2020b) reported the first case of glyphosate-resistant johnsongrass in Cordoba, Spain, in railroad and freeway populations that were routinely exposed to glyphosate, wherein reduced herbicide translocation led to 4.2 to 9 times greater resistance compared to a susceptible population. Several johnsongrass biotypes have evolved resistance to glyphosate as a result of intensive use, especially across vast soybean production areas in Argentina (Vila-Aiub et al. Reference Vila-Aiub, Balbi, Gundel, Ghersa and Powles2007). The glyphosate-resistant accessions studied by Vila-Aiub et al. (Reference Vila-Aiub, Balbi, Distefano, Fernandez, Hopp, Yu and Powles2011) had less herbicide absorption and less translocation to root and stem meristems. An analysis using single sequence repeat markers among 46 glyphosate-resistant johnsongrass accessions collected across Argentina showed no clear pattern of association with geographical origin, suggesting multiple origins of resistance (Fernández et al. Reference Fernández, de Haro, Distefano, Martinez, Lia, Papa, Olea, Tosto and Hopp2013).
PMGF from HR Johnsongrass
Johnsongrass is a highly self-pollinated species, but PMGF is possible between different johnsongrass biotypes. Maity and Bagavathiannan (Reference Maity and Bagavathiannan2021) studied PMGF from nicosulfuron-resistant johnsongrass to susceptible (pollen recipient) johnsongrass biotypes in Texas. They found PMGF as high as 13.7% at the shortest distance of 5 m, and up to 2.3% at the farthest studied distance of 50 m. These levels of outcrossing are sufficient for transferring herbicide resistance alleles between adjacent field populations. Even at distances farther than those studied by Maity and Bagavathiannan (Reference Maity and Bagavathiannan2021), PMGF can be greater than the rate of spontaneous mutation for conferring herbicide resistance in this species. Johnsongrass is also known to outcross with other compatible sorghum relatives across the agricultural landscape, with a potential for transferring herbicide resistance and other traits (Ohadi et al. Reference Ohadi, Hodnett, Rooney and Bagavathiannan2017). Despite the ploidy barriers between johnsongrass (2n = 40) and cultivated sorghum (2n = 20), PMGF has been reported between the two species (Arriola and Ellstrand Reference Arriola and Ellstrand1996; Hodnett et al. Reference Hodnett, Ohadi, Ace Pugh, Bagavathiannan and Rooney2019; Sias et al. Reference Sias, Hodnett, Rooney and Bagavathiannan2020; Subramanian et al. Reference Subramanian, Hodnett, Rooney and Bagavathiannan2021). Historical exchange of alleles between johnsongrass and sorghum in agricultural landscapes has been documented by Morrell et al. (Reference Morrell, Williams-Coplin, Lattu, Bowers, Chandler and Paterson2005). Arriola and Ellstrand (Reference Arriola and Ellstrand1997) found no fitness differences between the F1 johnsongrass (female) × sorghum hybrids and wild johnsongrass. Gene flow involving johnsongrass has practical significance when it acts as either a male or a female parent (Ohadi et al. Reference Ohadi, Hodnett, Rooney and Bagavathiannan2017).
Shattercane [S. bicolor (L.) Moench subsp. drummondii (Steud.) de Wet ex Davidse] (2n = 20) is a closely related species of sorghum present in sorghum production regions in the United States (Schmidt Reference Schmidt2011). Shattercane is thought to be a feral form of cultivated sorghum (Ejeta and Grenier Reference Ejeta, Grenier and Gressel2005) with a very high degree of genetic similarity with sorghum and sudangrass (S. bicolor ssp. drummondii), an annual forage sorghum species (Defelice Reference Defelice2006). Thus, the PMGF between shattercane × johnsongrass should be similar to that of sorghum × johnsongrass. The PMGF between sorghum (female) × johnsongrass was as high as 0.5% with certain sorghum genotypes and environments (Sias et al., unpublished data). Feral HR sorghum in agricultural landscapes may serve as conduits for the transfer of herbicide resistance and other traits between johnsongrass and sorghum in either direction (Ohadi et al. Reference Ohadi, Littlejohn, Mesgaran, Rooney and Bagavathiannan2018). Currently, sorghum cultivars with non-transgenic herbicide resistance (InzenTM sorghum with resistance to nicosulfuron; igrowthTM sorghum with resistance to imazamox; and DoubleTeamTM sorghum with resistance to quizalofop) are under commercial development. Likewise, PMGF between johnsongrass and shattercane or HR sorghum volunteers (or vice versa) in production fields can facilitate trait movement.
Rigid (Annual) Ryegrass
The genus Lolium belongs to the Poaceae family and includes eight diploid species with a chromosome number 2n = 14 (Table 1). These species originated in Europe, North Africa, and temperate Asia and are distributed throughout the temperate regions of the world (Bennett et al. Reference Bennett, Hayward and Marshall2000). The genus contains species such as L. multiflorum, L. perenne, and L. rigidum, which are obligate outcrossing and wind-pollinated (Matzrafi et al. Reference Matzrafi, Preston and Brunharo2021). Rigid ryegrass is the most widespread and troublesome weed in Australia and is currently ranked as the number one weed worldwide for its ability to evolve resistance to a number of herbicides with different SOAs (Busi et al. Reference Busi, Dayan, Francis, Goggin, Lerchl, Porri, Powles, Sun and Beckie2020; Heap Reference Heap2021).
Rigid Ryegrass Reproductive Biology
Rigid ryegrass is a self-incompatible, outcrossing species and a prolific pollen producer (Gill Reference Gill1996; McCraw and Spoor Reference McCraw and Spoor1983; Figure 6A). It has a high degree of genetic variability that helps it adapt to a range of climatic and edaphic conditions (Kloot Reference Kloot1983). In Australia, rigid ryegrass populations with large differences in phenological development have been documented, demonstrating adaptation to local environments (Gill et al. Reference Gill1995). It also exhibits genotypic plasticity (Gill et al. Reference Gill1995), creating the potential for PMGF that enables the spread of herbicide resistance traits (e.g., paraquat or glufosinate resistance in Lolium; Yu et al. Reference Yu, Cairns and Powles2004) or resistance to newly discovered herbicides (Brunton et al. Reference Brunton, Gill and Preston2021), favoring the evolution of multiple HR populations (Figure 6B).

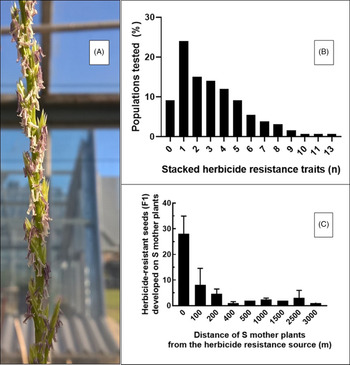
Figure 6. (A) Inflorescences of rigid ryegrass (Lolium rigidum) with extruded stamens (photo credit: Roberto Busi), (B) frequency (%) of tested rigid ryegrass populations (n = 600) exhibiting resistance to a number of herbicides, and (C) number of resistance seeds from herbicide-susceptible (S) rigid ryegrass mother plants placed at increasing distance from fields infested with herbicide-resistant rigid ryegrass.
Herbicide Resistance in Rigid Ryegrass
Rigid ryegrass is the most damaging weed in Australia, with an estimated cost of AUS$100 million annually (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). Herbicide-resistant rigid ryegrass infests millions of hectares of the southern Australian cropping region (Busi et al. Reference Busi, Beckie, Bates, Boyes, Davey, Huskins, Mock, Newman, Porri and Onofri2021; Busi and Beckie Reference Busi and Beckie2021).
Rigid ryegrass is an excellent example of the ability of a weed species to evolve resistance to herbicides with different SOAs (Heap Reference Heap2021). This is a consequence of persistent herbicide selection leading to a steady increase in the frequency of cross-resistance and multiple resistance to ALS- and ACCase- inhibiting herbicides (Heap and Knight Reference Heap and Knight1986; Owen et al. Reference Owen, Martinez and Powles2014; Figure 6B). In a recent study, Busi et al. (Reference Busi, Beckie, Bates, Boyes, Davey, Huskins, Mock, Newman, Porri and Onofri2021) showed that out of 600 rigid ryegrass populations tested across the Australian continent, only 9% were susceptible to all herbicides tested (Figure 6B). Conversely, more than 50% of the tested populations survived at least three different herbicides. One population of rigid ryegrass evolved resistance to at least 13 of the herbicides tested, including five different SOAs (ACCase, ALS, microtubule, lipid biosynthesis, and VLCFA inhibitors; Figure 6B). This confirms the formidable ability of rigid ryegrass to adapt and evolve resistance to herbicides (Heap Reference Heap2021) and the accumulation of multiple resistance traits in self-incompatible cross-pollinated individuals (i.e., multiple HR field populations).
PMGF from HR Rigid Ryegrass
Loureiro et al. (Reference Loureiro, Escorial and Chueca2016) studied PMGF in rigid ryegrass under both greenhouse and field conditions using a diclofop-methyl-resistant biotype as the pollen donor. The maximum PMGF was 56.1% in the greenhouse. Data from the field study were fit to an exponential decay model to predict gene flow at increasing distances. When the model was based on data from all recipient plants in the study, it predicted an average PMGF of 7.1% when the pollen donor and receptor plants were 0 m apart and a 50% reduction at 16.7 m from the pollen source regardless of the direction. When recipient plants were downwind of the pollen source, however, PMGF was 5.2% at 25 m, the farthest distance studied.
PMGF can occur over long distances in rigid ryegrass (4% at >3,000 m; Figure 6C) from large field sources infested by HR rigid ryegrass to isolated herbicide-susceptible population (Busi et al. Reference Busi, Yu, Barrett-Lennard and Powles2008). Thus, the transfer of HR alleles from HR rigid ryegrass field populations can occur between fields and result in increased frequency of herbicide-resistant populations in a region (Beckie et al. Reference Beckie, Busi, Bagavathiannan and Martin2019). In a subsequent study, this hypothesis was validated by studying the dynamics of PMGF from organic fields and neighboring conventional fields with persistent herbicide use. In organic fields, the frequency of HR rigid ryegrass (identical haplotypes) was much higher than could be explained by a background mutation frequency (Busi et al. Reference Busi, Michel, Powles and Délye2011). For example, the frequency of field resistance to the herbicide diclofop-methyl was 3-fold to 10-fold greater than expected in unselected and genetically isolated rigid ryegrass populations (Neve and Powles Reference Neve and Powles2005).
Wild Oats
Avena fatua originated in the western Mediterranean (Loskutov Reference Loskutov2008) and has been introduced to most temperate regions globally. The species is found in Europe, the Middle East, western Asia, and North America (Beckie et al. Reference Beckie, Francis and Hall2012; USDA-NRCS 2020). Avena fatua occurs in a variety of non-crop disturbed (ruderal) and cultivated habitats, and prefers clay or clay loam soils (Beckie et al. Reference Beckie, Francis and Hall2012). Avena sterilis (sterile wild oat) likely spread as a weed in small-grain cereals from the Fertile Crescent (Middle East) 3,000 to 4,000 yr ago to its present native range throughout the Mediterranean region and southern Europe (Rosentrater and Evers Reference Rosentrater and Evers2018). It was subsequently introduced into central and eastern Europe and other continents via seed contamination of wool and grain (Stace Reference Stace1997; Torner et al. Reference Torner, Gonzalez-Andujar and Fernandez-Quintanilla1991). Avena sterilis is well adapted to a wide range of climatic conditions and is commonly found in semi-arid, temperate, and sub-tropical regions globally; it occurs on all continents except Antarctica (Anonymous 2019; Floc’h Reference Floc’h, Groves and Castri1991; Holm et al. Reference Holm, Pancho, Herberger and Plucknett1979; O’Donnell et al. Reference O’Donnell, Adkins and Walker2002). The species can be found in cropland, pastures, vineyards, and ruderal areas; it tends to prefer heavier-textured soils compared with those favored by A. fatua (Stace Reference Stace1997). In addition, A. sterilis can grow under drier conditions than A. fatua (Fernández-Quintanilla et al. 1990; Thurston Reference Thurston1951).
Wild Oats Reproductive Biology
Avena fatua and A. sterilis have an annual life cycle and grow up to 1.5 m high (Anonymous 2019; Beckie et al. Reference Beckie, Francis and Hall2012). The open loose panicle of A. fatua is 10 to 40 cm long, with spikelets that have two glumes 1.8 to 2.5 cm long containing three florets (Figure 7 A and B). The florets separate through disarticulation of the rachillas segments, and each floret has a twisted awn 3 to 4 cm long (Figure 8; Bajwa et al. Reference Bajwa, Akhter, Iqbal, Peerzada, Hanif, Manalil, Hashim, Ali, Kebaso, Frimpong, Namubiru and Chauhan2017). The inflorescence of A. sterilis is an equilateral, pyramidal, or slightly one-sided loose panicle, 15 to 45 cm long and 8 to 25 cm wide (Stace Reference Stace1997). Spikelets are 1.7 to 4.5 cm long without the awns, each with two to five florets of which only the lowest has a basal scar, disarticulating above the glumes but not between the florets. Glumes are equal or subequal, and 2.4 to 5.0 cm long. The lemma is 1.5 to 4.0 cm long, with the uppermost (third seed) being awnless and the lower two (first and second seeds) having a 3 to 9 cm long bent and twisted dorsal awn (Ivens Reference Ivens1989). The caryopsis length is 10 mm with a width of 0.2 to 0.25 cm.

Figure 7. (A) Wild oat (Avena fatua): three florets/seeds and (B) wild oat mature plants in a crop canopy (photo by K.N. Harker, used with permission).

Figure 8. A wild oat panicle during anthesis with yellow anthers visible on some of the florets (photo credit: Breanne Tidemann).
Compared with A. fatua, the stem of A. sterilis is more prostrate, with more tillers at the maximum tillering stage. The panicles are lighter in weight because the spikelets have fewer and smaller florets, while the glume is longer, the awn is absent for the third seed, the spikelets are hard and do not break easily, and the seeds remain longer within the spikelet at maturity and are shed in units of 2 or 3 (Moss Reference Moss2015; Stace Reference Stace1997). A. fatua and A. sterilis are hexaploid, with a chromosome count of 2n = 42 and genome AACCDD (Bajwa et al. Reference Bajwa, Akhter, Iqbal, Peerzada, Hanif, Manalil, Hashim, Ali, Kebaso, Frimpong, Namubiru and Chauhan2017; Beckie et al. Reference Beckie, Francis and Hall2012).
Herbicide Resistance in Wild Oats
Herbicide resistance in A. fatua was first reported in Australia in 1985 and has since been reported in 17 countries, whereas resistance in A. sterilis has been documented in nine countries since first reported in 1989 (Heap Reference Heap2021). Most HR populations are resistant to ACCase- or ALS-inhibiting herbicides. The incidence of herbicide resistance in A. sterilis is significantly less than that of A. fatua because of lower relative abundance and more restricted global distribution. While herbicide resistance in A. sterilis remains limited to ACCase and ALS inhibitors and microtubule inhibitors, flamprop-methyl, A. fatua populations in Canada and the United States have also exhibited resistance to lipid synthesis inhibitors or cell elongation inhibitors, microtubule inhibitors, VLCFAE inhibitors, and PPO inhibitors (Heap Reference Heap2021; Mangin et al. Reference Mangin, Hall and Beckie2017; O’Donovan et al. Reference O’Donovan, Sharma, Harker, Maurice, Baig and Blackshaw1994). Five-way herbicide resistance was found in an A. fatua population from Manitoba, Canada (Mangin et al. Reference Mangin, Hall and Beckie2017), which is the only Avena species population reported to exhibit resistance to VLCFAE- or PPO-inhibiting herbicides. In 2018, the first and only cases of glyphosate resistance in A. fatua and A. sterilis were identified in a chickpea (Cicer arietinum L.) field in New South Wales, Australia (Heap Reference Heap2021; B. Chauhan pers. comm.). Although this biotype is not reported elsewhere, A. fatua was predicted to be at high risk of selection for glyphosate resistance in western Canada because of significant use of glyphosate (Beckie et al. Reference Beckie, Blackshaw, Low, Hall, Sauder, Martin, Brandt and Shirriff2013a).
The most recent (2014 to 2017) survey in western Canada identified HR A. fatua in 69% of 578 fields where samples were collected (Beckie et al. Reference Beckie, Shirriff, Leeson, Hall, Harker, Dokken-Bouchard and Brenzil2020), which was significantly higher than 44% of sampled fields in the previous survey (2007 to 2009; Beckie et al. Reference Beckie, Lozinski, Shirriff and Brenzil2013b). In the 2014 to 2017 survey, populations with resistance to ACCase inhibitors, ALS inhibitors, and both ACCase and ALS inhibitors occurred in 62%, 34%, and 27% of fields containing A. fatua, respectively (Beckie et al. Reference Beckie, Shirriff, Leeson, Hall, Harker, Dokken-Bouchard and Brenzil2020). There was a marked increase in resistance to ALS-inhibiting herbicides and resistance to both ACCase and ALS inhibitors in the most recent survey due to increased use of ALS inhibitors to manage ACCase inhibitor-resistant A. fatua populations (Beckie et al. Reference Beckie, Shirriff, Leeson, Hall, Harker, Dokken-Bouchard and Brenzil2020). In contrast, herbicide resistance in Avena species (A. fatua and A. sterilis) remained stable in Western Australia between 2005 and 2010, with the most recent survey of 128 populations in 2015 indicating resistance to ACCase inhibitors, ALS inhibitors, and both ACCase and ALS inhibitors in 48%, 2%, and 7% of populations, respectively (Owen and Powles Reference Owen and Powles2009, Reference Owen and Powles2016). A 2007 survey of 113 Avena species from New South Wales, Australia, found that 38% of populations were resistant to ACCase inhibitors, while no populations exhibited resistance to ALS inhibitors (Broster et al. Reference Broster, Koetz and Wu2011). In Greece, 81% of 104 A. sterilis populations in 2009 were ACCase inhibitor-resistant, whereas only 3% were ALS inhibitor-resistant (Travlos et al. Reference Travlos, Giannopolitis and Economou2011).
PMGF from HR Wild Oats
Both Avena species are highly self-pollinating. Flowering in A. sterilis occurs earlier than in A. fatua (Holm et al. Reference Holm, Pancho, Herberger and Plucknett1979; Stace Reference Stace1997). In A. fatua, anthesis within each spikelet occurs in order from the first to the third floret (Raju et al. Reference Raju, Jones and Ledingham1985). Some pollens emitted by anthers are intercepted by adjacent branches of the stigma, with embryo development occurring 24 to 40 h after pollination. Anthesis continues within a panicle for 4 to 14 d. Florets are normally chasmogamous vs. cleistogamous. Insects or wind have not been reported as significant vectors in pollination (Murray et al. Reference Murray, Morrison and Friesen2002).
There is a paucity of data regarding the extent of PMGF in A. sterilis under field conditions, although rates are expected to be similar among Avena species (Bajwa et al. Reference Bajwa, Akhter, Iqbal, Peerzada, Hanif, Manalil, Hashim, Ali, Kebaso, Frimpong, Namubiru and Chauhan2017; Cavan et al. Reference Cavan, Biss and Moss1998; Darmency and Uludag Reference Darmency and Uludag2018). The PMGF is limited in A. fatua (Sharma and Vanden Born Reference Sharma and Vanden Born1978). Using ACCase inhibitor resistance as a marker, PMGF for A. fatua in a non-crop environment in western Canada ranged between 0% and 12.3% (mean of 5.2%; Murray et al. Reference Murray, Morrison and Friesen2002). Distance from the pollen source (maximum 0.56 m) was a significant factor only for the high-density planting arrangement in flax (Linum usitatissimum L.). Therefore, intraspecific pollination is substantially reduced within crop canopies. In spring wheat (Triticum aestivum L.), PMGF in A. fatua at low density (19 plants m−2) and high density (37 plants m−2) was 0.08% and 0.05%, respectively; in the less competitive flax, PMGF averaged 0.07% and 0.16%, respectively (Murray et al. Reference Murray, Morrison and Friesen2002). These results indicate that PMGF does contribute to the evolution and spread of herbicide resistance in A. fatua populations, although it is of minor importance compared with seed-mediated gene flow (Murray et al. Reference Murray, Morrison and Friesen2002).
Hybridization can occur between Avena species. For example, A. sterilis hybridizes with A. sterilis sub-species and other Avena species such as A. fatua (Cavan et al. Reference Cavan, Biss and Moss1998), A. sativa L. (Mariot et al. Reference Mariot, Sereno, Federizzi and Carvalho1999; Sereno-Tavares et al. Reference Sereno-Tavares, Bodanese-Zanettini and de Carvalho1995), and A. nuda L. (Yu et al. Reference Yu, Chen and Liu1998). A. fatua can create fertile hybrids when crossed with A. sterilis (Cavan et al. Reference Cavan, Biss and Moss1998). Sterile hybrids have been recorded between A. fatua and A. strigose Schreb., A. barbata Pott ex Link, A. magna H.C. Murphy & Terrell, and A. murphyi Ladiz. (Rajhathy and Thomas Reference Rajhathy and Thomas1974).
Avena fatua can produce up to 1,070 seeds per plant in non-crop conditions; however, 20 to 150 seeds per plant are more common in competitive environments (Beckie et al., Reference Beckie, Francis and Hall2012). Avena fatua seed shatters before most crops mature. For example, studies have reported that 30% to 70% of A. fatua seed shattered before wheat harvest in western Canada (Burton et al. Reference Burton, Beckie, Willenborg, Shirtliffe, Schoenau and Johnson2016, Reference Burton, Beckie, Willenborg, Shirtliffe, Schoenau and Johnson2017; Shirtliffe et al. Reference Shirtliffe, Entz and Van Acker2000; Tidemann et al. Reference Tidemann, Hall, Harker, Beckie, Johnson and Stevenson2017). This variability in percent seed shatter is likely affected by genetic heterogeneity and phenotypic plasticity. Seed shatter from panicles may begin 2 to 3 wk before the typical wheat harvest in India (Balyan et al. Reference Balyan, Malik, Panwar and Singh1991). Seed dispersal primarily occurs near the parent plant (Beckie et al. Reference Beckie, Francis and Hall2012); however, combine harvesters can spread seeds over distances up to 145 m (Shirtliffe and Entz Reference Shirtliffe and Entz2005). In addition, seed dispersal occurs via birds or other animals, although the scale of anthropogenic dispersal via grain seedlot mixture, hay, or farm equipment is considerably greater (Stace Reference Stace1997; Terry Reference Terry1984). Avena sterilis can produce up to 200 seeds plant−1, although substantially less seed is produced under inter- or intraspecific competition (Fernández-Quintanilla et al. Reference Fernandez-Quintanilla, Navarrete, Andujar, Fernandez and Sanchez1986).
Summary and Research Needs
Reviewing the literature on reproductive biology, HR biotypes, and PMGF studies of the economically important HR grass weeds, barnyardgrass, creeping bentgrass, Italian ryegrass, johnsongrass, rigid ryegrass, and wild oats, confirms that transfer of HR alleles is possible. Rigid ryegrass and Italian ryegrass are self-incompatible, outcrossing species with the potential for long-distance PMGF and transfer of herbicide resistance alleles. In addition, Italian ryegrass readily hybridizes with rigid ryegrass as well as perennial ryegrass and produces hybrids, thereby increasing chances of PMGF and transfer of resistance alleles in Lolium species.
Genetically engineered glyphosate-resistant feral hybrids between creeping bentgrass and compatible species have been identified multiple times in situ in Oregon and Idaho. The plants were found in agricultural fields, irrigation canals, and non-agricultural sites. Due to its reproductive biology, widespread occurrence, and the number of sympatric, compatible species, the risk of PMGF and transfer of HR alleles in creeping bentgrass is high. Experience has shown that the HR trait in creeping bentgrass cannot be contained once it is released, nor is the appearance of the gene likely to decline over time because no fitness penalty from the gene has been documented. Glyphosate was used for the control of weeds along irrigation systems but is no longer effective for the control of glyphosate-resistant creeping bentgrass, which can clog waterways and slow water movement (Figure 2). Further studies are needed to monitor the resistance gene over time to determine its spread and stability in hybrids. Johnsongrass is a highly self-pollinating species; however, PMGF up to 2.3% has been reported in Texas from nicosulfuron-resistant to -susceptible johnsongrass at 50 m from the pollen source. With respect to herbicide resistance traits, PMGF between johnsongrass, shattercane, and HR sorghum poses challenges for HR trait sustainability. Therefore, research is needed to rotate sorghum with other crops to reduce PMGF.
Barnyardgrass is an autogamous species with a low level of PMGF, thus seed movement would most likely contribute more to the landscape-level spread of herbicide resistance compared to PMGF. Herbicide resistance in wild oats is a global issue, but cross- or multiple-herbicide resistance is most extensive in the northern Great Plains of North America. Gene flow studies in wild oats have confirmed that PMGF is possible; however, because both wild oat species (A. fatua and A. sterilis) are highly self-pollinating, the spread of herbicide resistance alleles has been mediated primarily by natural or anthropogenic seed dispersal. Therefore, research is needed to reduce seed-mediated gene flow in barnyardgrass and wild oats by developing best management practices such as harvest weed seed destruction.
The scientific literature is limited regarding the reproductive biology of grass weed species. Therefore, research is needed to investigate pollen viability affected by varying levels of temperature, moisture, and relative humidity, flowering synchrony among and between economically important grass weeds, and the possibility of PMGF. Landscape-level PMGF has been studied in rigid ryegrass and creeping bentgrass. This type of study is needed for other HR grass weed species such as barnyardgrass, Italian ryegrass, johnsongrass, and others in different environments. Studies of PMGF are usually focused on moderately to highly outcrossing weed species and neglect low outcrossing species. Dense populations of the latter species can elevate the importance of PMGF relative to seed movement that needs to be investigated. Climate change and unfavorable weather events such as flood, tornado, and high wind are likely to bring further shifts in weed species occurrence and distribution; therefore, research is needed on how climate change may affect PMGF in economically important grass weed species. For example, higher temperatures during pollination and greater precipitation variability in the future may exacerbate or mitigate the contribution of PMGF to movement of herbicide resistance alleles among weed populations.
Acknowledgments
We thank Dr. Maria Zapiola for comments and suggestions for improvement of the creeping bentgrass section. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. No conflicts of interest have been declared.