Article contents
Superconducting TaC nanoparticle-containing ceramic nanocomposites thermally transformed from mixed Ta and aromatic molecule precursors
Published online by Cambridge University Press: 07 August 2017
Abstract
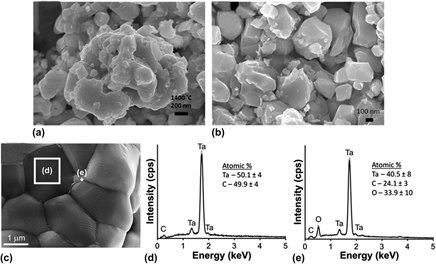
We report the structure and synthesis approach for obtaining a ceramic nanocomposite pellet comprising ∼50 nm-sized TaC nanoparticles. A mixture of Ta metal powder and the carbon precursor 1,2,4,5-tetraphenylethynyl benzene, pelletized by vacuum pressing at 131 MPa, on further thermal treatment with Ar at 1400 °C yields such a ceramic composite. On air oxidation, the TaC nanoparticles are converted to Ta2O5 nanoparticles at 760 °C. Hardness measurements revealed that the composite exhibited a global hardness in the range of 1.23–1.57 GPa. However, nanoindentation studies showed that, locally, hardness of the TaC nanoparticles (∼15 GPa) approached that of the densified TaC ceramic. Superconducting studies of the pellet consistently exhibited two transitions with T c values of 10 K and 8.5 K, respectively, that corresponded to bulk TaC and to a component of unknown origin. The results discuss the morphological and constitutional characterizations of the TaC nanoparticle-containing composite.
Keywords
- Type
- Invited Articles
- Information
- Journal of Materials Research , Volume 32 , Issue 17: Focus Issue: Achieving Superior Ceramics and Coating Properties through Innovative Processing , 14 September 2017 , pp. 3353 - 3361
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
National Research Council Postdoctoral Fellow.
Contributing Editor: Xiaowei Yin
References
REFERENCES
- 5
- Cited by





