Introduction
Biodiversity has value for the global community, but conservation of habitats and species can involve non-trivial costs for local communities in developing countries (Balmford et al., Reference Balmford, Bruner, Cooper, Costanza, Farber and Green2002). These costs can preclude the behaviour change needed to achieve conservation objectives, raising the need for incentives to help communities overcome the cost of conservation. Conservation International, through its Conservation Stewards Program, uses conservation agreements to create such incentives in more than 15 countries, to protect 1.5 million ha of habitat and improve the livelihoods of 35,000 people. These initiatives have also been scaled up to regional and national programmes, conserving an additional 1.7 million ha and benefiting more than 240,000 people. The model offers direct incentives, with conservation investors providing a negotiated benefit package in return for conservation actions by communities (Simpson & Sedjo, Reference Simpson and Sedjo1996; Ferraro, Reference Ferraro2001; Ferraro & Kiss, Reference Ferraro and Kiss2002; Niesten et al., Reference Niesten, Zurita and Banks2010). Thus, the agreements link funders (governments, bilateral agencies, companies, foundations, individuals, etc.) to resource owners whose decisions influence conservation outcomes (Milne & Niesten, Reference Milne and Niesten2009).
Direct incentives offered under conservation agreements can take the form of cash disbursement to individuals and/or community funds, thereby converging with direct payments for conservation (Ferraro & Kiss, Reference Ferraro and Kiss2002; Milne & Niesten, Reference Milne and Niesten2009; Clements et al., Reference Clements, Rainey, An, Rours, Tan and Thong2013). Like such approaches, the efficacy of agreements hinges on verified compliance with commitments. Conservation agreements also share this quid pro quo character with payments for ecosystem services (Wunder, Reference Wunder2005, Reference Wunder2007; Engel et al., Reference Engel, Pagiola and Wunder2008). Conservation agreements are a means to make a wide variety of interventions (payments for ecosystem services, co-management of protected areas, environmental offsets, and others) tangible and attractive for communities.
Here we describe the conservation agreement model and its application in Colombia's Amazon region to conserve freshwater fish species. To demonstrate the impacts of these agreements, we summarize fish population trends revealed through 8 years of monitoring, noting the challenge of linking interventions to outcomes (Baylis et al., Reference Baylis, Honey-Rosés, Börner, Corbera, Ezzine-de-Blas and Ferraro2016). We show how ecological monitoring results are shaping plans for sustainable extraction and we conclude by noting implications for scaling up.
The conservation agreement model
The conservation agreement model consists of four phases: feasibility analysis; community engagement; agreement design and negotiation with resource users; and implementation (CSP, 2016). The feasibility analysis informs implementers whether an agreement may be suitable for a given site. If so, the implementer approaches the resource users to introduce the model and gauge community interest in developing an agreement. If resource users explicitly express a desire to proceed, joint design of the conservation agreement begins.
The agreement specifies rights and responsibilities of the parties involved, conservation commitments of resource users, benefits provided by the implementer, and penalties for non-compliance (CSP, 2016), resembling payment for ecosystem services as defined in Wunder (Reference Wunder2005). Most agreements involve two parties, with one undertaking conservation actions and the other providing benefits and technical support for those actions, but other parties, such as government, may be involved. Community commitments in the agreement are based on the conservation objective; they can include direct behaviour change, such as spatial restraints on shifting cultivation or desisting from illegal hunting or fishing, and/or actions to reduce external pressure, such as patrolling to deter poachers. Benefit packages are designed to address the opportunity cost of conservation: the value of foregone resource use, such as income lost by not expanding crop fields, plus the cost of conservation actions such as time spent patrolling. Benefits can include cash payments to individuals, often as wages for patrolling, and/or investments that provide group benefits, such as small-scale irrigation infrastructure.
A key feature of conservation agreements is that benefits depend on compliance with commitments (Milne & Niesten, Reference Milne and Niesten2009). Graduated penalties are defined jointly by implementers and communities as part of agreement design. Sanctions usually start with an admonishment letter requesting corrective actions. If non-compliance persists, the benefit package is reduced temporarily, with restoration of full benefits once needed actions are taken. Finally, in the event of continuous breaches, implementers terminate the agreement and then must decide whether to pursue an alternative strategy, such as intensified law enforcement, or redirect scarce conservation funds to initiatives elsewhere.
Often, an initial agreement is signed for 1 year; if it works well the parties renegotiate and renew for another year. After 3–5 years, implementers explore sustainable financing options for a long-term agreement. Options include trust funds, payments for ecosystem services (e.g. carbon sequestration, watershed protection), and private sector partnerships (e.g. social and environmental offsets, or green enterprises). Some countries offer scope for government programmes that support conservation and poverty alleviation through conservation agreements, as in Ecuador's Programa Socio Bosque (de Koning et al., Reference de Koning, Aguiñaga, Bravo, Chiu, Lascano, Lozada and Suárez2011) or the Programa de Incentivos por Conservación, Manejo Integral y Servicios de Bosque in the Department of Pando, Bolivia (Espinoza et al., Reference Espinoza, Malky and Bruner2015). Sustainability also involves improved local governance capacity to reduce reliance on technical support. Once sustainable finance is in place, a long-term agreement can be signed.
Monitoring conservation agreements
Monitoring is essential to verify that conservation agreements achieve environmental and development objectives. Monitoring is also needed to verify agreement compliance, and the presence of meaningful monitoring can itself be a driver of behaviour change (Sommerville et al., Reference Sommerville, Milner-Gulland, Rahajaharison and Jones2010). The model's emphasis on monitoring responds to increasing calls for more rigorous impact evaluation in the conservation arena (Kleiman et al., Reference Kleiman, Reading, Miller, Clark, Scott and Robinson2000; Ferraro & Pattanayak, Reference Ferraro and Pattanayak2006; Fisher et al., Reference Fisher, Balmford, Ferraro, Glew, Mascia, Naidoo and Ricketts2014; Baylis et al., Reference Baylis, Honey-Rosés, Börner, Corbera, Ezzine-de-Blas and Ferraro2016). A well-designed conservation agreement includes attention to evaluation such that monitoring results facilitate clear attribution of impacts to interventions (Ferraro & Hanauer, Reference Ferraro and Hanauer2014). This provides confidence that funds are well spent, and is essential for convincing funding sources and policy makers of the value of scaling up the approach.
Monitoring compliance with conservation commitments is often performed by the implementers. Typically implementers can observe compliance directly, as they provide technical assistance and project follow-up. In addition, implementers should devise systems that encourage community members to report infractions by others, and ensure that sanctions are applied. Regular reviews of the agreement by the implementers and communities permit examination of accomplishments and analysis of why some conservation commitments may go unfulfilled.
To ensure objectivity, biodiversity monitoring is led usually by third parties other than the implementer or resource users. Each agreement defines biodiversity baselines and measurable conservation goals, such as number of hectares conserved or species populations maintained/increased, and annual monitoring tracks progress on these goals. Monitoring results are used to fine-tune agreement terms over time. Involving resource users in biodiversity monitoring efforts has helped empower and engage communities in several agreement sites (e.g. Cambodia, China, Colombia and Guatemala).
Whenever possible, socio-economic monitoring is also conducted by third parties. As with biodiversity monitoring, a socio-economic baseline established in the first year of the agreement is followed by annual monitoring. The purpose is to understand changing socio-economic conditions of resource users and track community perceptions about the agreement and the benefits provided, as these will influence compliance and agreement robustness. With an eye to longer-term sustainable resource management, socio-economic monitoring also considers institutional development and governance capacity. Finally, socio-economic monitoring informs renegotiations to ensure that benefits respond to community priorities and that resource users are satisfied with the design and implementation of the agreement.
Monitoring of compliance is distinct from monitoring of ecological and socio-economic impacts, although ideally verified compliance can be linked to measurable positive impacts. However, the credibility of asserted links must be examined carefully during project evaluation. A strong record of community compliance with an agreement may not yield desired outcomes if, for example, impacts of climate change or intense pressure from outsiders overwhelm local management actions. Likewise, care must be taken to ensure that positive impacts can be attributed legitimately to behaviour change brought about by a conservation agreement.
Conservation agreements in the Colombian Amazon
In 1998 Conservation International's Colombia programme began working in the corregimiento of La Pedrera, in the Amazonas Department bordering Brazil (Colombia is divided administratively into Departments, which comprise municipalities and corregimientos; these in turn may be divided into districts called veredas). During 2002–2004 the programme conducted a participatory environmental assessment of the Lower Caqueta river basin, with three Indigenous Reserves and two veredas. Building on previous ecological research, the assessment informed management plans for legally recognized community territories in 2005. While preparing these plans, communities identified El Francisco Creek and the lakes of Puerto Caimán, Bacurí, Del Monte and Taraira as priorities for local well-being (Fig. 1). By 2008 the management plans included resource-use rules and designated conservation areas, but the communities lacked the means to carry out management activities. Outsiders continued to fish pirarucú Arapaima gigas and silver arowana Osteoglossum bicirrhosum within the conservation areas, using destructive methods and harvesting fish below the legal minimum catch-size; local fishers, seeing the rules violated with impunity, followed suit.
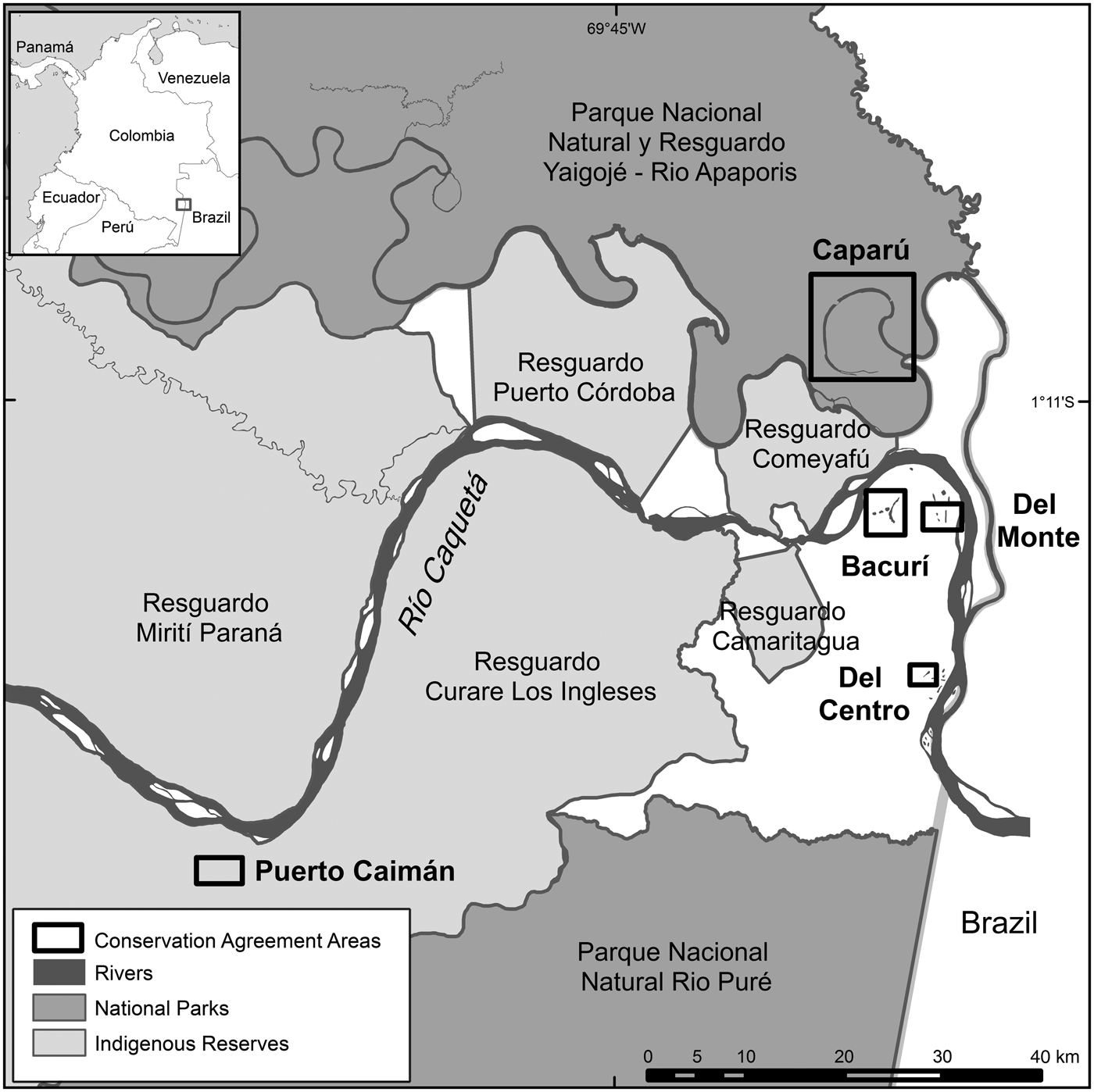
Fig. 1 Location of the sites in La Pedrera, Amazonas, Colombia, where Conservation International established conservation agreements with local communities to protect forest areas and two threatened fish species, the pirarucú Arapaima gigas and the arowana Osteoglossum bicirrhosum.
The arowana exhibits paternal mouth brooding, in which the male cares for the eggs and fry. This species congregates in small schools, sought by fishers to extract males carrying fry in their mouths. The fry are transported to Bogotá for export as ornamental fish to Europe, Asia and the United States. In Colombia an estimated 195,000–1,150,000 fingerlings are sold per year (Mancera-Rodríguez & Álvarez-León, Reference Mancera-Rodríguez and Álvarez-León2008), and this market pressure has caused local extinctions in several areas of the Amazon (Duque et al., Reference Duque, López-Casas, García-Vega and Bravo-Osorio2008; Proterra, 2012). The pirarucú is the world's second-largest freshwater fish, with records of individuals exceeding 3 m in length and 200 kg in weight. Like the arowana, it is an oral incubator. The pirarucú has also been driven to local extinction in several areas of the Putumayo basin and the Colombian Amazon as a result of its high commercial value, especially in sales to restaurants, and has been listed in Appendix II of CITES (2017) since 1992 (Duque et al., Reference Duque, López-Casas, García-Vega and Bravo-Osorio2008; Proterra, 2012; Fishbase, 2015; IUCN, 2016). Although Arapaima gigas is categorized as Data Deficient on the IUCN Red List (World Conservation Monitoring Centre, 1996) and the global status of Osteoglossum bicirrhosum has not been evaluated, at the national level in Colombia both are listed as Vulnerable based on IUCN criteria (Ministry of Environment, Housing and Territorial Development, 2010).
Both arowana and pirarucú live and breed in lakes in the project area but migrate during the rainy season when the lakes and rivers are connected (Duque et al., Reference Duque, López-Casas, García-Vega and Bravo-Osorio2008; Moreno-Arias & Moreno-Arias, Reference Moreno-Arias and Moreno-Arias2010; López-López et al., Reference López-López, Moreno-Arias and Gulfo2011; Proterra, 2012). Migration can complicate monitoring and species-focused incentive arrangements for conservation in marine or aquatic systems (Begossi et al., Reference Begossi, May, Lopes, Oliveira, da Vinha and Silvano2011; Bladon et al., Reference Bladon, Short, Mohammed and Milner-Gulland2016), but the agreements in the Colombian Amazon benefit from a focus on lakes that serve as primary breeding areas. By 2008 communities were consistently reporting increasing scarcity of fish in lakes, rivers and streams as a result of overexploitation by local fishers and others from surrounding communities, La Pedrera, and Brazil. Destructive fishing methods (e.g. fine-mesh netting and poison) also affected other species, such as black caimans Melanosuchus niger, giant otters Pteronura brasiliensis, giant river turtles Podocnemis expansa and c. 20 other fish species that are important sources of protein for local communities.
During 2008–2009, Conservation International established conservation agreements to conserve pirarucú, arowana and other species with seven communities (c. 500 people) in the three Indigenous Reserves and two veredas. The communities were selected based on proximity to key lakes and creeks and desire to improve implementation of territorial management plans. The agreements seek to conserve 193,870 ha of freshwater ecosystems and surrounding forest, by providing incentives to offset the opportunity cost of foregoing destructive fishing practices and undertaking activities to enforce regulations and conservation areas defined in management plans.
The agreements were signed by leaders in each community following extensive consultations with all members, witnessed by representatives from indigenous peoples’ organizations linked to the Reserves. Each community also had a natural resource committee that was instrumental firstly in developing the management plans, and then in designing conservation agreement terms focused on capacity to implement the plans. Capacity-building of the committees themselves was also included as an agreement benefit.
The communities committed to stop fishing pirarucú and arowana in protected lakes and creeks, forbid fishing during the spawning season, use only artisanal fishing gear, establish fishing quotas for other species, and participate in surveillance activities to prevent outsiders from fishing in conservation areas. Restrictions on fishing by community members reflect voluntary commitments to uphold self-imposed regulations in the management plans, and vigilance efforts against outsiders reinforce indigenous rights to control territorial access. Patrolling teams consist of three community rangers from different families. Each patrolling campaign lasts 30 days. If outsiders (or insiders) are found fishing for pirarucú or arowana in protected lakes or creeks, patrollers inform the natural resource committee of their Indigenous Reserve or vereda, which then reports the incident to local police and environmental authorities. Patrollers also participate in pirarucú and arowana monitoring, such that the methodology we describe below incorporates their knowledge.
In exchange for these efforts, community rangers are paid the equivalent of the prevailing wage rate. Patrol team members rotate monthly to ensure that nearly every family in each community can participate. However, community rangers do not have the authority to arrest and detain people and do not carry weapons, so they require support from police and military to enforce restrictions against outsiders.
The benefit package includes funds for natural resource committees, to ensure they have the means to report problems to authorities. Additional benefits of the agreements include investment in community leadership and governance, such as training in administration, conflict resolution and planning. Communities also enjoy further advantages beyond the direct benefits, as lake protection supports a range of fish species that are vital to food security, and community patrolling also strengthens territorial rights. The value of this last consideration is further enhanced in the Indigenous Reserves, where lakes protected by agreements are also sacred sites.
The agreements explicitly define graduated sanctions to be applied in case of non-compliance. Each community defined internal sanctions for members who breach the agreements, based on the severity of the infraction. Minor infractions (e.g. using project equipment for non-project travel) result in a warning initially, and after a second offence transgressors are penalized by a 10% wage reduction. If non-compliance persists, the offender is suspended from the project for 6 months. Serious infractions (e.g. shirking on patrolling duties) result in a 20% wage reduction. A second offence leads to a 6-month suspension from the project. Sanctions for very serious infractions (e.g. taking pirarucú and arowana from protected lakes) include expelling the transgressor from the project for 1 year.
Conservation International applies external sanctions only when communities do not apply internal penalties. A community that does not comply with its commitments loses 10% of its annual benefit package; these funds can be recovered if the breach is resolved. Upon a second breach, a community forfeits 20% of the annual payment, and the agreement is terminated after a third breach. The communities can also penalize the implementer for failure to deliver technical support or benefits on time: on the first occasion Conservation International must pay USD 90 to the community fund, the second time USD 180, and the third time USD 270. Sanctions are decided on and applied during monthly meetings between the communities and Conservation International.
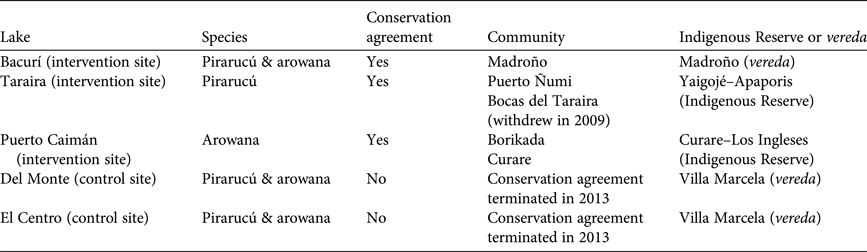
The initial agreements involved seven communities distributed among three Indigenous Reserves and two veredas: Borikada and Curare (Curare–Los Ingleses Indigenous Reserve), Camaritagua (Camaritagua Indigenous Reserve), Puerto Ñumi and Bocas del Taraira (Yaigojé–Apaporis Indigenous Reserve), and the veredas of Madroño and Villa Marcela. After the first year, Bocas del Taraira withdrew from the programme to avoid conflict with a neighbouring community where an influential fisher continued to ignore harvest restrictions in Taraira Lake. In 2013 Conservation International terminated the agreement with Villa Marcela because of persistent harvesting and selling of pirarucú, despite several warnings and community meetings. Table 1 summarizes the communities and lakes covered by the project, as well as lakes no longer covered by the project but examined for purposes of comparison.
Table 1 Project context for conservation agreements between Conservation International and communities in the Colombian Amazon (Fig. 1), to protect forest areas and two threatened fish species, the pirarucú Arapaima gigas and the arowana Osteoglossum bicirrhosum, including details of two control sites.
Monitoring impacts of the Colombian conservation agreements
Impact monitoring of the agreements sought to examine three principal issues. Firstly, are the agreements producing the desired biodiversity conservation outcomes, as indicated by trends in pirarucú and arowana populations? Secondly, are the agreements improving the lives of people in the communities, as reflected in socio-economic indicators? Thirdly, are the outcomes sustainable, as captured by metrics relating to community resilience to ecological and economic shocks? Here we focus on ecological results to examine whether conservation objectives are being achieved, and to assess long-term sustainable resource use options.
The National University of Colombia, through its Amazonian Research Institute, and Conservation International developed the initial ecological monitoring protocol, informed by community knowledge of the ecosystems. The biodiversity baseline was established in 2008, focusing on the lakes and excluding El Francisco Creek, as there is no robust monitoring framework for measuring pirarucú or arowana in rivers. Annual third-party monitoring was then conducted by the University of Antioquia, focused on the lakes of Puerto Caimán (Curare–Los Ingleses), Bacurí (Madroño) and Taraira (Yaigojé–Apaporis). In 2013, river levels were unusually high; continuous connectivity between rivers and lakes precludes reliable counts, so monitoring was foregone at all sites that year. In 2015 the monitoring effort also examined Del Monte and El Centro lakes (Villa Marcela), not covered by agreements, for comparison with lakes covered by the project. (Although the Villa Marcela agreement was terminated in 2013, the community consented to monitoring in Del Monte in 2014, and both Del Monte and El Centro in 2015.) Since 2013 Del Monte and El Centro lakes have served as control sites, as per impact evaluation practice (Ferraro & Hanauer, Reference Ferraro and Hanauer2014). Ideally project monitoring would have incorporated control sites from the outset, and strictly speaking the way the two non-project lakes came to be included does not conform to best practice for randomized controls (Ferraro & Pattanayak, Reference Ferraro and Pattanayak2006). However, the impracticality of ideal monitoring and evaluation scenarios is well known (Baylis et al., Reference Baylis, Honey-Rosés, Börner, Corbera, Ezzine-de-Blas and Ferraro2016). Given the realities of implementing conservation in practice, treating Del Monte and El Centro as comparison sites in a natural quasi-experiment illustrates a practical approach that permits inferences regarding project impacts.
The monitoring team, with technical support from the Sinchi Institute and the University of Antioquia, adapted the methodology developed by Castello (Reference Castello2001, Reference Castello2004) for monitoring pirarucú, which consists of counting individuals as they surface to breathe. Trained community members observe lake surfaces from canoes 100 m apart, and count the number of pirarucú surfacing to breathe in front of them in 30-minute intervals. As adult pirarucú surface every 15–20 minutes, the monitors must count the number of sightings and judge whether they have previously counted the same individual. Juvenile pirarucú are counted only during the first 20 minutes, as they breathe more frequently than the adults. The result is a count of the total population in a lake, but the margin of error remains to be determined by further research. Castello (Reference Castello2004) found that the results from this methodology are closely correlated (coefficient of 0.98) with total population estimates based on extrapolation from individual tagging and recapture records.
The initial monitoring approach for arowana was the same as that for pirarucú, but this proved unsuitable, as arowana are known to swim rapidly in schools near the surface at the lake periphery. This can easily lead to double-counting, and counts can differ by observer, depending on monitoring skills (Moreno-Arias & Moreno-Arias, Reference Moreno-Arias and Moreno-Arias2010). The methodology used since 2011 is based on the only documented arowana monitoring experience in the Amazon basin, in Pacaya–Samiria in Peru (Bodmer et al., Reference Bodmer, Puertas, Pérez, Ríos, Escobedo and Dosantos2006). Initially the new approach involved community members in canoes surveying lake edges during moonless nights. The monitors illuminated the water on the lake edge and counted the schools, recording numbers of adults and juveniles in each school (López-López et al., Reference López-López, Moreno-Arias and Gulfo2011). As of 2014, based on reports from community members that substantial numbers of arowana congregated in the middle of the lakes, the monitoring frame was extended to central lake areas.
Results from monitoring of pirarucú populations show clearly positive trends in the intervention sites (Table 2). The estimated population of pirarucú has increased substantially in Bacurí and Taraira, the two lakes protected continuously since 2008, from 11 to 199 individuals and 278 to 821 individuals, respectively (Taraira Lake is protected under the agreement with Puerto Ñumi even though the Bocas de Taraira community withdrew from the agreement in 2009). Even in Del Monte, where the agreement ended in 2013, the number of juveniles showed a substantial increase in 2015. In contrast, the population in El Centro appears to be stagnating, at best, and may be in decline, probably because it is most vulnerable to illegal fishing because of its location near the border with Brazil.
Table 2 Population trends of pirarucú in four lakes in the project area in the Colombian Amazon (Fig. 1) during 2009–2015.
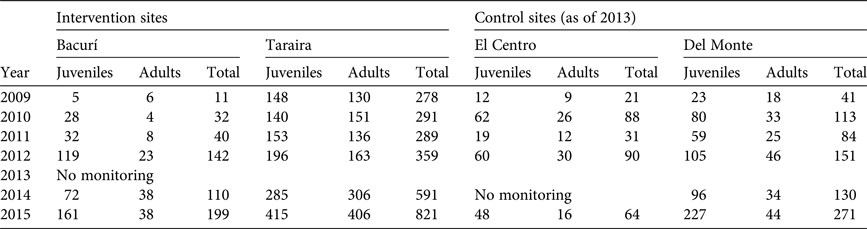
Similarly, the results of arowana monitoring indicate positive trends in the protected lakes, with pronounced increases in estimated populations from 2014 to 2015 in Puerto Caimán and Bacurí (Table 3). In the lakes of El Centro and Del Monte, where agreements were discontinued in 2013, arowana populations have crashed, consistent with observations of intensive harvesting pressure. Combined with the figures for pirarucú noted above, these trends suggest that incentives provided to communities under conservation agreements helped improve protection and management of key fish species in the project sites.
Table 3 Population trends of arowana in four lakes in the project area in the Colombian Amazon (Fig. 1) during 2010–2015.
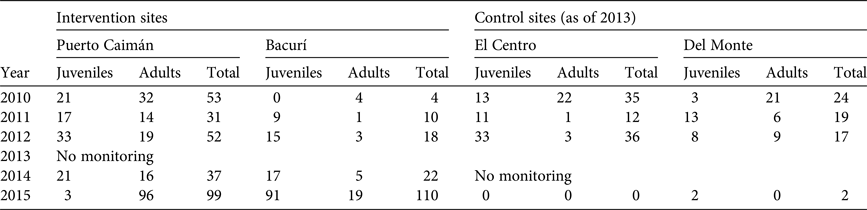
Tables 2 and 3 suggest different dynamics in the Del Monte and El Centro control sites; without intervention the pirarucú population is declining in El Centro but increasing in Del Monte, whereas arowana populations have crashed at both sites. Geography is the key factor: El Centro is in a sparsely inhabited area that is readily accessible to illegal fishers from Brazil as well as Colombia. Routes to Del Monte pass through inhabited areas and near a national forest reserve, resulting in a greater likelihood of fishers being seen and reported to authorities. Therefore El Centro is subject to greater pressure. Moreover, Del Monte is near other pirarucú habitat, and this species is known to migrate between floodplain habitats, especially during periods with high water levels (Fernandez, Reference Fernandez1997; Arantes et al., Reference Arantes, Castello, Cetra and Schilling2013). Thus, the Del Monte population probably benefits from enhanced protection at Bacurí through both vigilance and law enforcement in the general area as well as biological spillover. Arowana populations crashed at both sites because low initial levels made them particularly vulnerable to any resumption of harvesting.
These monitoring data warrant some caution. Community monitoring raises questions about skills and accuracy, and about possible motivations to overstate positive project outcomes. Guidance and supervision from third-party university experts mitigates these risks. However, impact evaluation with respect to freshwater fish species raises various technical complications, including mobility of the resource, time lags between intervention and observable impact, and the role of confounding factors such as climatic variation, disease, and predator population dynamics (Reid et al., Reference Reid, Contreras MacBeath and Csatádi2013; Adams et al., Reference Adams, Setterfield, Douglas, Kennard and Ferdinands2015). Therefore, relationships between conservation actions as verified by compliance monitoring, observed numbers based on ecological impact monitoring, and actual population remain subject to further research and refinement.
Nevertheless, despite methodological challenges, the results indicate that extreme population declines reported by communities prior to 2008 have been replaced by increasing trends in protected lakes. Contrasted with local extinctions observed elsewhere in the Putumayo basin and the Colombian Amazon, these results strongly suggest that arowana and pirarucú numbers are increasing as a result of the conservation agreements. Monthly community reports also note increasing presence of other species, particularly river turtles and giant otters.
Sustainable extraction
Increases in arowana and pirarucú populations motivated an analysis of the potential for sustainable extraction. Sustainable extraction rates depend on abundance, particularly of mature individuals larger than the minimum legal size for capture: 70 cm for arowana and 150 cm for pirarucú in Colombia (Gómez et al., Reference Gómez, Torres and Rengifo2013). Experience in Brazil's Mamirauá Sustainable Reserve and Peru's Pacaya–Samiria Reserve suggests that extraction rates of 5–10% of adult individuals in a population of pirarucú may be viable (Viana et al., Reference Viana, Castello, Damasceno, Amaral, Estupinán and Arantes2007; Gómez et al., Reference Gómez, Torres and Rengifo2013).
No defined methodology exists for estimating sustainable extraction rates of arowana fry. For this analysis we assumed the same rates as for pirarucú, recognizing that this may not reflect the actual potential for fry extraction, compounded by uncertainties regarding the methodology for counting arowana. Population figures reported in Table 3, assuming extraction rates of 5–10% for fry, suggest that Puerto Caimán cannot be harvested, whereas Bacurí could yield 4–9 individuals. Based on these results, harvesting arowana fry in the protected lakes is inadvisable, given the low numbers as well as year-on-year variability. Although arowana population trends in intervention sites appear to be positive, the populations have yet to recover from 2 decades of intensive harvesting.
Prospects for sustainable harvesting appear to be more promising for pirarucú, particularly given the greater confidence in the methodology for monitoring population trends. Assuming a 5–10% extraction rate for 2015 populations (Table 2), Bacurí could yield 2–4 mature adults, and Taraira 20–40 mature adults. Gomes (Reference Gomes2012) offers further guidance on determining sustainable extraction rates for pirarucú based on population densities in 58 lakes in Mamirauá (Brazil), with density ranges characterized as very low (0.04–1.09 individuals per ha), low (1.21–3.53 individuals per ha), medium (3.78–8.41 individuals per ha) and high (9.39–45.66 individuals per ha). As numerous factors affect population density, including habitat characteristics, life cycles, and intensity of flooding pulses, Gomes (Reference Gomes2012) posits that sustainable harvesting requires at least medium density.
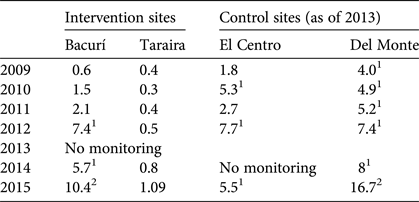
Densities of pirarucú in the lakes monitored by the conservation agreement project in the Colombian Amazon are in Table 4. The population density is much lower in Taraira than in other sites because the lake is 40–64 times larger in size, so even with a much larger population the density remains low. Only two lakes had a high population density, in only 1 year: Bacurí and Del Monte in 2015. Medium population density was recorded consistently in Del Monte, and in several years in Bacurí and El Centro, but the trends are not monotonically increasing. The population densities (Table 4) suggest that harvesting pirarucú in the protected lakes would be premature.
Table 4 Population densities (individuals per ha) of pirarucú in four lakes in the project area in the Colombian Amazon (Fig. 1) during 2009–2015.
1 Medium density
2 High density
A conservative option is to defer harvesting until densities have stabilized at high levels. A second measure to consider is working with community members to identify management zones within the lakes, such that localized population densities can inform harvesting decisions while protection of breeding areas is strengthened (Begossi et al., Reference Begossi, May, Lopes, Oliveira, da Vinha and Silvano2011; Arlinghaus et al., Reference Arlinghaus, Lorenzen, Johnson, Cooke, Cow and Craig2016). For instance, shallow portions of lakes or areas close to concentrations of predators are likely to have lower densities, and including them in density averaged over the entire lake may understate the health of the population. Other potential sustainable management measures include prohibiting harvesting during breeding periods and setting an appropriate minimum catch size to ensure that individuals breed at least once before capture (Castello, Reference Castello2001, Reference Castello2004; Gómez et al., Reference Gómez, Torres and Rengifo2013; Arlinghaus et al., Reference Arlinghaus, Lorenzen, Johnson, Cooke, Cow and Craig2016).
Discussion
The Colombia agreements seek to strengthen community resource governance, promoting self-determination while enhancing the overall context for socio-economic development. With respect to biodiversity objectives, key aspects of governance relate to respecting management rules and protecting resources against outside pressures. To monitor effectiveness of agreements, periodic assessments are conducted with the resource users. The Colombia project team participates in biannual community assemblies to review compliance. The team also participates frequently in discussions in the Maloca (ancestral long house used for community meetings by Amazonian indigenous groups, particularly in Colombia and Brazil), where people feel more comfortable speaking candidly than in formal household interviews. These interactions have also reinforced social control mechanisms, as people are aware that non-compliance could jeopardize the agreements. Together, increasing governance capacity and social pressure reflect institutional development that facilitates common property management of what was previously an open access resource (Agrawal, Reference Agrawal2001; Ostrom, Reference Ostrom2005). The incentives provided under the conservation agreements can be viewed as a way to overcome financial and other hurdles that previously prevented such institutional development.
Frequent communication between implementers and resource users and extensive participation of communities in ecological monitoring efforts mean that people are aware of the population trends of pirarucú and arowana. As they see these populations recovering, community desire to resume harvesting of these economically important species is increasing. Therefore, long-term conservation impact will require continued vigilance, strong social management structures, and sustainable harvesting regimes based on evidence-based determination of viable extraction rates (Begossi et al., Reference Begossi, May, Lopes, Oliveira, da Vinha and Silvano2011; Arlinghaus et al., Reference Arlinghaus, Lorenzen, Johnson, Cooke, Cow and Craig2016).
Despite increasing numbers of arowana and pirarucú, sustainable harvesting does not yet appear to be viable. However, orienting monitoring, analysis and discussions with communities around determining sustainable harvesting rates facilitates definition of shared goals on population targets and eventual harvest management regimes. A critical condition for sustainable extraction of arowana will be overcoming the challenges to measuring population status. Moreover, monitoring methods for both species must respond adequately to other factors that can influence fish populations (e.g. climate change), to demonstrate the link between agreement compliance and conservation outcomes (Ficke et al., Reference Ficke, Myrick and Hansen2007; Adams et al., Reference Adams, Setterfield, Douglas, Kennard and Ferdinands2015; Baylis et al., Reference Baylis, Honey-Rosés, Börner, Corbera, Ezzine-de-Blas and Ferraro2016). Sustainable extraction will also require effective vigilance activities and compliance monitoring, given the risks that community members may neglect harvest restrictions or that outsiders may take advantage of opportunities created by opening of lakes to fishing activities. This need highlights the importance of socio-economic monitoring that captures increase in community governance capacity, including capacity to conduct compliance monitoring (Agrawal, Reference Agrawal2001; Ostrom, Reference Ostrom2005; Cronkleton et al., Reference Cronkleton, Bray and Medina2011), as a precondition for sustainable harvesting.
Conservation agreements are often part of a larger strategy. In the Colombian Amazon, the agreements will not succeed without support from local authorities. Community rangers can inform outsiders that pirarucú and arowana are off limits, but they do not have the legal authority to apprehend transgressors or confiscate fishing gear, and doing so would put their lives at risk. Therefore, community patrols must be reinforced by support from Colombian authorities, including the environmental police. One option being explored is to secure additional financial and policy support from the regional environmental authority, CorpoAmazonia, to continue implementing the current agreements and expand the initiative to other communities facing similar challenges.
Expanded use of conservation agreements with support from a government agency would require monitoring of compliance and impacts on a larger scale. Monitoring frameworks must be adapted to the scale of implementation, and larger scales involve simplification and reliance on remote sensing technologies at the expense of local specificity (Vincent, Reference Vincent2016). Impact evaluation at scale involves challenges related to choice of indicators and effects of heterogeneity (Baylis et al., Reference Baylis, Honey-Rosés, Börner, Corbera, Ezzine-de-Blas and Ferraro2016; Vincent, Reference Vincent2016), but monitoring to track fine-scale ecological and socio-economic impacts at many sites will be costly. The conservation agreements in Colombia show that an effective biodiversity monitoring framework capable of capturing local detail is essential to determine whether an agreement is accomplishing its conservation goals, especially if sustainable harvesting of focal species is an ultimate objective.
The Colombian Amazon experience suggests that meaningful monitoring in large-scale programmes would benefit from site-specific partnerships between communities, universities and research centres, and implementing organizations. Experience in other national and large-scale payment for ecosystem services programmes using conservation agreements confirms the value of such multi-stakeholder collaboration (de Koning et al., Reference de Koning, Aguiñaga, Bravo, Chiu, Lascano, Lozada and Suárez2011; Espinoza et al., Reference Espinoza, Malky and Bruner2015). Although replication and scale-up are needed to increase overall impact, local-level focus remains essential to ensure demonstrable outcomes.
Acknowledgements
The following supported monitoring and documentation of project impacts in La Pedrera: Mulago Foundation, CorpoAmazonía, Mitsubishi Corporation Foundation for the Americas, Toyota Environmental Activities Grant Program, Swift Family Foundation, and the Conservation Agreements Private Partnership Platform (funded by the Global Environmental Facility through the United Nations Environment Programme). We thank the communities of Borikada, Curare, Camaritagua, Puerto Ñumi, Bocas del Taraira, Madroño and Villa Marcela, and the teams who worked with them to collect information. We also thank two anonymous reviewers for thorough and thoughtful input that greatly improved the article.
Author contributions
MM supervised the research effort and drafted sections of the article. EP compiled data and background information. EN conceptualized and wrote the article. All authors reviewed and provided substantive comment on the manuscript and approved the final version.
Biographical sketches
Margarita Mora leads the implementation of conservation agreements in Africa, Asia and Latin America. Erwin Palacios’s research interests include large vertebrate abundance, hunting patterns in Colombian Amazonia and community-based conservation initiatives. Eduard Niesten’s research interests include incentive-based approaches and innovative conservation finance.









