Article contents
Influence of Carbon Structure and Physical Properties on The Corrosion Behavior in Carbon Based Air Electrodes for Zinc Air Batteries
Published online by Cambridge University Press: 10 February 2011
Extract
Rechargeable zinc-air batteries are a long run time solution for portable electronics. Cells with a nominal voltage of 1.05 V have a specific energy of 169 Wh kg−1 and aenergy density of 219 Wh L1. This cell is capable of delivering 16 – 18 Ah of capacity at 2 A (16.7 mA cm−2) discharge rate. However, the cycle life of these cells is artificially shortened because of the generation of carbon dioxide in the alkaline electrolyte during the cycling of the cell. The carbon dioxide has the effect of removing the OH− (equation 1) needed by the anode
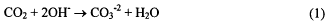
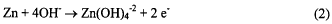
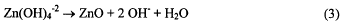
half reaction (equations 2 and 3). The need for hydroxide is demonstrated in equation 2.
There is literature evidence [1,2] that the CO2 was the result of corrosion of the carbon matrix of the air electrode. We report here the results of our investigation of this corrosion reaction and what properties of the carbon contribute to the corrosion reaction rate.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1998
References
REFERENCES
- 4
- Cited by




