The lumen of the large intestine is an intensely proteolytic environment. Recent studies have suggested that metabolites derived from bacterial handling of proteins are implicated in the pathogenesis of various diseases. Both ammonia, derived from proteolysis, as well as ureolysis and phenolic compounds, formed out of aromatic amino acids, are important in the pathogenesis of the uraemic syndrome (Vanholder et al. Reference Vanholder, Argilés and Baurmeister2001). In vitro studies also suggest a role for metabolites from protein fermentation in the pathogenesis of colon cancer (Visek, Reference Visek1978; Cummings et al. Reference Cummings, Hill, Bone, Branch and Jenkins1979; Corpet et al. Reference Corpet, Yin, Zhang, Remesy, Stamp, Medline, Thompson, Bruce and Archer1995).
Fermentation of carbohydrates may cause a decrease in the production of toxic metabolites from protein fermentation, including ammonia and phenolic compounds, through various mechanisms (Vince & Burridge, Reference Vince and Burridge1980; Cummings & Bingham, Reference Cummings and Bingham1987; Weber et al. Reference Weber, Banwell, Fresard and Cummings1987; MacFarlane et al. Reference MacFarlane, Gibson and Cummings1992). However, studies addressing this topic lead to different results. This is due to the fact that the excretion of ammonia and phenols is influenced by a number of factors besides carbohydrate fermentation, which cannot all be controlled (Cummings et al. Reference Cummings, Hill, Bone, Branch and Jenkins1979; MacFarlane et al. Reference MacFarlane, Gibson and Cummings1992).
In the present study, an attempt was made to study protein fermentation in a direct way using safe and non-radioactive markers. Both the fate of ammonia present in the colon and the bacterial production of phenolic compounds were studied by means of substrates labelled with stable isotopes. Stimulation of carbohydrate fermentation was induced either by inclusion of inulin in the test meal or by addition of inulin to the daily diet, allowing us to distinguish between changes induced by the actual presence of a fermentable carbohydrate and effects caused by a 4-week dietary intervention.
Inulin-type fructans are fructose polymers of different chain length, which escape digestion in the small intestine due to the β-configuration of the fructosidic linkages. As a result, about 85–90 % of ingested carbohydrate is likely to reach the colon, where it is readily fermented (Roberfroid, Reference Roberfroid1999; Cummings et al. Reference Cummings, Macfarlane and Englyst2001). Direct effects attributed to carbohydrate fermentation include an enhanced bacterial growth and thus N uptake, a decrease in pH due to SCFA production and a reduction of the enzymes necessary for protein breakdown through a mechanism called catabolite repression (Vince & Burridge, Reference Vince and Burridge1980; Cummings & Bingham, Reference Cummings and Bingham1987; Weber et al. Reference Weber, Banwell, Fresard and Cummings1987; MacFarlane et al. Reference MacFarlane, Gibson and Cummings1992). Inulin is also a prebiotic, which means that it is a non-digestible dietary supplement that modifies the balance of the intestinal microflora, stimulating the growth and/or activity of beneficial organisms for human health and suppressing potentially deleterious bacteria (Cummings et al. Reference Cummings, Macfarlane and Englyst2001; Roberfroid, Reference Roberfroid2001). The selective growth stimulation of bifidobacteria in the colonic microbiota by the inulin-type fructans was observed both in vitro and in human studies (Roberfroid, Reference Roberfroid2001). Microbial activity has been studied in continuous-culture systems as well as in faecal slurries. The activities of enzymes such as azoreductase, nitroreductase, β-glucuronidase and β-glucosidase, which are thought to favour carcinogen formation in the colon, were evaluated (Cummings et al. Reference Cummings, Macfarlane and Englyst2001). A decrease in enzyme activity was not always observed in dietary intervention studies in human subjects. However, studies of faecal enzyme activities are notoriously difficult to interpret and the in vitro system may well be a better model of what is going on in the proximal gut. Furthermore, whether changes in enzyme activity translate into a changed product formation depends on the substrate availability, pH and a number of other factors (Cummings et al. Reference Cummings, Macfarlane and Englyst2001). There is a need for more human nutrition studies and/or new markers to investigate the interaction of prebiotics with colon carcinogenesis, since various types of animal models consistently demonstrate a reduced risk in experimentally induced carcinogenesis processes with inulin-type fructans (Van Loo et al. Reference Van Loo, Cummings and Delzenne1999).
In the present study, we investigated the production and fate of bacterial metabolites using two substrates labelled with stable isotopes; lactose [15N, 15N]ureide (LU) as a source of labelled ammonia and egg proteins intrinsically labelled with [2H4]tyrosine as a precursor of [2H4]p-cresol. Both ammonia and phenolic compounds are believed to be carcinogenic (Visek, Reference Visek1978; Cummings et al. Reference Cummings, Hill, Bone, Branch and Jenkins1979; Corpet et al. Reference Corpet, Yin, Zhang, Remesy, Stamp, Medline, Thompson, Bruce and Archer1995).
Materials and methods
Experimental design
It was decided to study as many variables as possible in the present study. The laborious work did not allow us to study large cohorts of volunteers. Therefore, the present study has to be considered as a pilot study and further studies are necessary to confirm the findings.
The first part of the study involved the evaluation of the excretion of the labelled markers in twelve healthy volunteers (four men and eight women) upon administration of inulin together with the test meal. The volunteers performed a 24 h urine collection and a 3 d stool collection once after ingestion of the pancake test meal containing labelled LU and egg proteins intrinsically labelled with [2H4]tyrosine and once again after ingestion of the pancake meal containing an additional 5 g Raftilin HP® (Orafti, Tienen, Belgium).
In the second part of the study, the influence of daily administration of inulin during 4 weeks was investigated in seven healthy volunteers (two men and five women). They received 5 g Raftilin HP® three times per d for 1 month, except on the days the tests were performed. All volunteers collected urine for 24 h and all stools during 3 d after ingestion of the test meal. They did this once before the start of the dietary intervention, once after they had been taking inulin for 1 week, once again after 1 month and once more 1 week after having stopped the intake of inulin.
The volunteers kept diet records the week before the first test and in between the following experiments in order to allow a qualitative comparison of dietary intakes. They were allowed to eat their usual diets, but were urged to keep a constant macronutrient composition.
All subjects ingested 1 g unlabelled LU the evening before each test and then fasted overnight.
Substrates
The marker molecules were administered in a pancake test meal, with a total energy content of 1369 kJ (327 kcal), containing 19 g protein, 6 g fat and 27 g carbohydrate. The batter of the pancake was made with two whole eggs intrinsically labelled with [2H4]tyrosine. These eggs were obtained by feeding laying hens feed supplemented with [2H5]-phenylalanine (3 g/kg; mol percent 98 %; Euriso-Top, Saint Aubin, France) (labels at all ring-positions). Part of the ingested [2H5]phenylalanine was hydroxylated to [2H4]-tyrosine by the hens' metabolism and, as a consequence, both marker molecules were incorporated in the egg proteins. The [2H4]tyrosine content of each egg was determined by means of GC-MS (trace GC-MS; Thermofinnigan, San José, CA, USA).
Labelled LU (75 mg; synthesised according to the method of Schoorl as modified by Hofmann (Reference Hofmann1931)) with [15N, 15N]urea obtained from Euriso-Top was added to the pancake batter. Unlabelled LU (1 g) was ingested on the evening before the test in order to induce the proper enzyme activity in the colonic bacteria (Wutzke et al. Reference Wutzke, Heine, Plath, Leitzmann, Radke, Mohr, Richter, Gulzow and Hobusch1997).
185 kBq of [3H]polyethylene glycol ([3H]PEG) (NEN Life Science Products Inc., Boston, MA, USA) was added to the test meal as an inert radiolabelled transit marker in order to correct for oro-anal transit time (Krag et al. Reference Krag, Krag and Lenz1975).
The inulin source in the study was Raftilin HP® (Orafti, Tienen, Belgium), a linear β (2,1)-linked fructose polymer, terminated by a sucrose residue, purified from chicory root. According to the supplier, Raftilin HP® contains >99 % inulin with a degree of polymerisation ranging between 5 and 60 and < 0·5 % glucose, fructose and sucrose.
Sample collection and storage
Urine samples were collected in plastic containers to which 1 g neomycin was added in order to prevent bacterial growth. All volunteers were asked to void before consumption of the test meal and this urine collection was used for measurement of the basal N content and 15N enrichment. The natural urinary content of [2H4]p-cresol and [2H4]phenol was zero. After ingestion of the pancake, urine was collected during 1 d in different fractions: 0–6 h, 6–10 h, 10–24 h. After measurement of the volume, samples were taken and stored at − 20°C until analysis.
The volunteers also performed a 72 h stool collection. Date and time of voiding of stools were noted in a diary. The stools were weighed immediately after voiding and all stools collected on the same day were combined and homogenised before further analysis.
Fresh faecal samples ( ± 10 g for every day of the collection) were diluted tenfold with sterile pyrogen-free water. Homogenisation was performed with a tissue-homogeniser at 20 MHz (M. Zipperer GmbH, Staufen, Germany) for 1 min. The homogenate was ultracentrifuged at 25 000 g for 120 min (MR22i; Jouan, St-Herblain, France) and the supernatant fraction was subsequently filtered through a 0·2 μm syringe filter (Supor Acrodisc 32, Gelman Sciences, Ann Arbor, MI, USA) in order to discard the ultimate faecal rests and the bacteria. The final filtrate was used for determination of total phenol, total p-cresol, [2H4]p-cresol and [2H4]phenol (Evenepoel et al. Reference Evenepoel, Claus, Geypens, Hiele, Geboes, Rutgeerts and Ghoos1999; Geypens et al. Reference Geypens, Claus, Gorris, Evenepoel, Luypaerts, Rutgeerts and Ghoos1999).
Another faecal sample of 10 g for each day of the collection was immediately freeze-dried. These samples were used for separation into the bacterial, fibre and soluble fractions.
The remainder of the stool collections was diluted with 500 g sterile pyrogen-free water. After homogenisation, a sample of known weight was removed and freeze-dried. The dried material was weighed again and samples were taken for analysis of N and radioactivity.
Analyses
Determination of total nitrogen content and 15N enrichment in urine, faeces and bacterial pellets
Total N content and 15N enrichment of urine and faeces were measured using an elemental analyser (ANCA-SL; PDZ Europa Ltd, Northwich, Cheshire, UK) coupled with both a thermal conductor detector (PDZ Europa Ltd) and a stable isotope ratio mass spectrometer (20-20 IRMS; PDZ Europa Ltd). A known volume of urine (15 μl), faeces (freeze-dried, about 7 mg) or bacterial pellets (freeze-dried, about 3 mg) was oxidised in the presence of O2 at 1000°C. The combustion products thereafter passed through a second furnace containing Cu at 600°C where excess O2 was absorbed and nitrogen oxides were reduced to elemental N. Total N content was measured by means of a thermal conductor detector, whereas the 15N enrichment was determined by means of an isotope ratio mass spectrometer detector, coupled to the combustion unit of the elemental analyser.
The results for the urinary and faecal collections were expressed as total N (g) and percentage of administered dose 15N. Total N in the bacterial fraction was expressed in mg/g bacteria and 15N excretion was expressed in ng/mg.
Determination of urinary and faecal total p-cresol, total phenol, [2H4]p-cresol and [2H4]phenol
The concentrations of p-cresol, phenol, [2H4]p-cresol and [2H4]phenol were determined by means of GC-MS (Trace GC MS; Thermofinnigan, San José, CA, USA) according to the procedure described by Geypens et al. (Reference Geypens, Claus, Gorris, Evenepoel, Luypaerts, Rutgeerts and Ghoos1999). Briefly, both for urinary and faecal analysis, samples with a volume of 950 μl were taken. After adjusting the pH to 1 with H2SO4 (Merck KgaA, Darmstadt, Germany), the solution was heated for 30 min at 90°C in order to deproteinise and hydrolyse the conjugated phenols. After cooling down to room temperature, 50 μl of 2,6 dimethylphenol (20 mg/100 ml water) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was added as internal standard and the phenols were extracted with 1 ml ethyl acetate (Merck KgaA, Darmstadt, Germany). Thereafter, the ethyl acetate layer was dried over anhydrous sodium sulfate and 0·5 μl of this solution was analysed on a GC-MS.
The results were expressed as total p-cresol and phenol (mg) excreted in urine or in faeces. For [2H4]p-cresol and [2H4]phenol, the results were expressed as percentage of administered dose of [2H4]tyrosine.
Finally, it was decided to report urinary excretion of labelled N separately for the 0–6 h and the 6–24 h urine collections, to allow for differentiation between small-bowel and colonic events. On the other hand, there was no need to examine the urinary excretion of labelled phenolic compounds in the separate fractions, since both [2H4]p-cresol and [2H4]-phenol can only be derived from colonic bacterial metabolism.
Determination of faecal [3H]polyethylene glycol
The [3H]PEG content in stool was measured by liquid-scintillation counting (Packard Tricarb Liquid Scintillation Spectrometer, model 3375; Packard Instruments Inc., Downers Grove, IL, USA) after oxidation to 3H-labelled water (Packard Sample Oxidiser, model 306; Packard Instruments Inc.).
3H-labelled water contents in stool were expressed as percentage of administered dose recovered over 72 h and were used to correct for gastrointestinal transit by dividing the cumulative percentage of administered dose of 15N recovered over 72 h by the cumulative percentage of administered dose of 3H recovered over 72 h (i.e. corrected faecal 15N excretion). Supplementation of inulin to the daily diet may influence oro-anal transit, whereas a single dose of inulin will probably not. Nevertheless, oro-anal transit time may vary on a day-to-day basis. Inclusion of the [3H]PEG marker allows us to distinguish between a true change in faecal 15N excretion and a change caused by an alteration of transit time (Krag et al. Reference Krag, Krag and Lenz1975).
Separation of the faeces into different fractions
A sample of 250 mg freeze-dried faecal material was taken and thoroughly mixed for 10 min with 15 ml formylsaline (1 % formol in 0·9 % saline) and 0·15 ml of 10 % sodium lauryl sulfate. The mixture was filtered through a 150 μm filter (Varian Inc., Palo Alto, CA, USA) under vacuum and the residue was washed with 15 ml formyl saline, shaken and filtered again. This procedure was repeated twice and, as a result, about 90 ml filtrate was obtained.
The fraction remaining on top of the filter was the fibre fraction and was kept for further analysis. The filtrate was ultracentrifuged at 25 000 g for 36 min. The supernatant fraction, containing the water-soluble compounds, was discarded, while the sediment, containing the bacteria, was dissolved in 2 ml sterile pyrogen-free water. After weighing, the sediment was freeze-dried and the dried material was weighed again (Stephen & Cummings, Reference Stephen and Cummings1980a). Samples of the remaining fibre fraction and the freeze-dried bacterial fraction were examined for the presence of bacteria.
Examination of the faecal fractions
Nine paired samples from the fibre fraction and the bacterial fraction were examined for the presence of bacteria using light microscopy and the Gram stain technique. Air-dried, heat-fixed smears of all samples were stained first with crystal violet (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and then with iodine solution (Sigma-Aldrich Chemie GmbH). Following rinsing with an alcohol–acetone mixture, staining with Safranin (Sigma-Aldrich Chemie GmbH) was performed. The smears were then analysed in a blinded manner without prior knowledge of the origin of the samples.
Additionally, three paired samples were examined with PCR analysis. Classical DNA extraction was performed by adding 0·5 ml proteinase K solution (0·2 mg/ml) (Sigma-Aldrich Chemie GmbH). Samples were incubated overnight at 55°C in a moving water-bath. The proteinase K solution was subsequently inactivated by heating up to 95°C for 10 min. The actual extraction was performed by the application of phenol and chloroform–isoamylalcohol (24:1) (Sigma-Aldrich Chemie GmbH). Afterwards, DNA was precipitated in a 10 m-ammonium acetate solution (Sigma-Aldrich Chemie GmbH; VWR, Haasrode, Belgium) mixed with 100 % ethanol at − 20°C. Following two episodes of ultracentrifugation (at 40 800 g) and washing, DNA pellets were dried and dissolved in 50 μl water and finally stored at − 20°C.
For PCR analysis, 2 μl DNA of each sample, 10 μl PCR Master Mix (Promega Corporation, Madison, WI, USA), 0·2 μl Jenssen1 primer and 0·2 μl Jenssen2 primer (Eurogentec, Seraing, Belgium) were dissolved in 7·6 μl distilled water. Both primers are complementary to conserved regions of the 16S and 23S gene, bordering spacer regions between those genes, with repeated appearance (of variable length) on the chromosome. 16S and 23S are important respectively for the small and large subunit of bacterial ribosomes. The sequence for Jenssen1 primer is 5′-GAA-GTC-GTA-ACA-ACG-3′. The sequence for Jenssen2 primer is 5′-CAA-GGC-ATC-CAC-CGT-3′. PCR analysis consisted of heating for 10 min at 94°C, twenty-five cycles (94°C, 1 min; 55°C, 4 min; 72°C, 2 min), followed by 7 min at 72°C and cooling until 4°C. PCR products were visualised using agarose gel electrophoresis. The gel was prepared using 0·25 agarose and 25 ml Tris Acetate EDTA (TAE) buffer (40 mm-tris(hydroxymethyl)-aminomethane, 10 mm-sodium acetate anhydrous, 1 mm-EDTA). The solution was boiled until the agarose was dissolved and poured into a mall for coagulation. Samples for analysis were introduced in the gel and the gel was placed in an electrophoresis device for 30 min at 90 V.
Statistical analysis
All results were expressed as median plus interquartile range and non-parametric statistical analysis was used (Friedman ANOVA and Wilcoxon test; P is significant at α = 0·05; Statistica 6.0, Statsoft Inc. 1984–2001, Tulsa, OK, USA).
Results
Examination of the faecal fractions
Light microscopy confirmed the presence of large numbers of bacterial colonies in all the samples of the bacterial fraction. In the fibrous residue occasional bacteria or small colonies were visualised in only two out of nine samples.
PCR analysis confirmed the presence of bacterial DNA in the three samples of the bacterial fraction and was negative in two out of three samples of the fibre fraction.
Effect of the presence of inulin in the meal (n 12)
Total nitrogen and 15N enrichment in urine
Table 1 shows the results of total N and 15N excretion in urine after simultaneous administration of inulin and labelled LU in twelve volunteers. The daily urinary total N excretion amounted to 10 g in baseline conditions as well as in the presence of inulin. On the other hand, a statistically significantly lower 24 h cumulative urinary excretion of the label was found when inulin was present in the test meal (Wilcoxon test; P = 0·02). Urine had been collected in 0–6 h, 6–10 h and 10–24 h fractions, since this allowed us to investigate different events during transit separately: the 0–6 h urine collection is said to refer to passage through the small bowel, whereas the later collections would reflect colonic events. The 6–24 h urine fraction was collected in two fractions (6–10 h and 10–24 h) in order not to obscure a possible early effect of inulin. However, we observed a decrease in urinary 15N excretion after inulin intake from the 6–10 h collection onwards. Therefore, it was reasonable to combine both fractions and to present the data as a 6–24 h collection. Upon ingestion of inulin, a statistically significant decrease in urinary excretion of the label was found in the 6–24 h collection (Wilcoxon test; P = 0·04).
Table 1 Effect of inclusion of inulin into the test meal on urinary excretion of nitrogen and 15N in twelve subjects (Medians and interquartile ranges (IQR))

* Wilcoxon test, α = 0·05.
Total nitrogen and 15N enrichment in faeces
Complete faecal collections were obtained in nine out of twelve volunteers. Stool weight, faecal dry weight, total N excretion and N density were not influenced by inclusion of inulin into the test meal (Table 2). However, a statistically significantly higher 72 h cumulative faecal excretion of 15N was found when inulin was present in the test meal. This difference was not caused by an increase in transit, since no higher recovery of [3H]PEG was found when inulin was present in the test meal (Wilcoxon test; NS). It was only when inulin was present in the meal that reliable measurements of both total N and labelled N were found in the bacterial pellets (Table 2).
Table 2 Effect of inclusion of inulin into the test meal on faecal parameters, including excretion of nitrogen and 15N, and on the excretion of total and labelled nitrogen in the bacterial fraction in nine subjects (Medians and interquartile ranges (IQR))
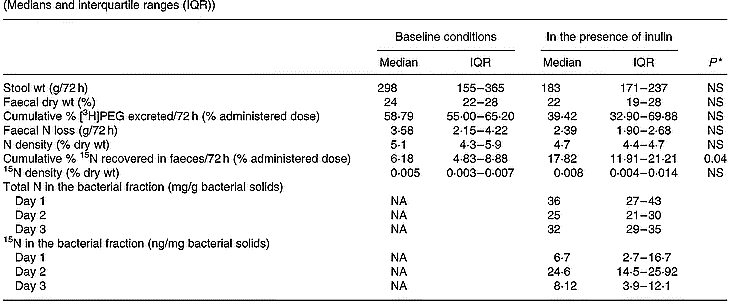
PEG, polyethylene glycol; NA, not applicable.
* Wilcoxon test, α = 0·05.
Total 15N excretion
The decrease in urinary 15N excretion was compensated by an increase in faecal excretion of the labelled compound (Tables 1 and 2). No difference in total excretion of the marker was found (53·13 (interquartile range 47·26–62·05) % in baseline conditions v. 54·42 (interquartile range 47·13–56·20) % when inulin was present in the meal; Wilcoxon test; NS), which means that addition of inulin did not cause any difference in retention of the label in the human body N pool.
Total and labelled phenol and p-cresol in urine
As was expected, the urinary excretion of total phenol and p-cresol was not influenced by a single administration of 5 g inulin (Table 3). However, a tendency towards a decreased urinary excretion of [2H4]phenol was noted upon addition of inulin, and a statistically significantly lower production of [2H4]p-cresol was found upon simultaneous administration of inulin.
Table 3 Effect of inclusion of inulin into the test meal on the excretion of labelled and unlabelled phenol and p-cresol in twelve subjects (Medians and interquartile ranges (IQR))

* Wilcoxon test, α = 0·05.
Effect of daily administration of inulin during 4 weeks (n 7)
Total nitrogen and 15N enrichment in urine
The urinary excretion of total N and 15N during long-term administration of inulin is shown in Table 4. The daily urinary N excretion was similar throughout the study and varied around 10 g/24 h. In addition, no statistically significant difference in 24 h cumulative urinary excretion of the labelled N was found at any occasion.
Table 4 Effect of long-term administration of inulin on the urinary excretion of nitrogen and 15N in seven subjects (Medians and interquartile ranges (IQR))
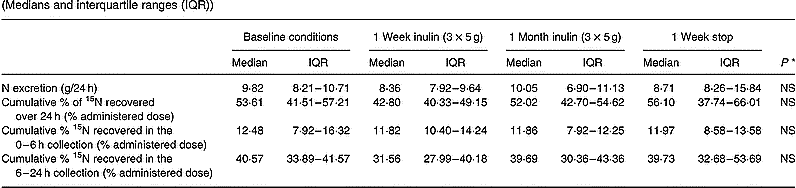
* Friedman ANOVA, α = 0·05.
Total nitrogen and 15N enrichment in faeces
Upon inclusion of 3 × 5 g inulin per d in the diet, no statistically significant difference in stool weight, faecal dry weight or oro-anal transit was found (Table 5). Total N excretion and excretion of the labelled marker were not influenced by the diet intervention. The contents of total N and labelled N in the bacterial fraction were below the limit of reliable detection of the ANCA-SL elemental analyser (PDZ Europa Ltd).
Table 5 Effect of long-term administration of inulin on the faecal parameters, excretion of nitrogen and 15N in faeces, and total 15N excretion (i.e. sum of cumulative recovery/72 h in urine and faeces) in seven subjects (Medians and interquartile ranges (IQR))

PEG, polyethylene glycol.
* Friedman ANOVA, α = 0·05.
Total 15N excretion
Since the total excretion of the marker (i.e. cumulative urinary excretion/24 h+cumulative faecal excretion/72 h) was similar in each of the test conditions, there was no difference in retention of the label in the human body N pool (i.e. 100 – total excretion of the marker) (Table 5).
Total and labelled phenol and p-cresol in urine and faeces
In this part of the study we also evaluated faecal excretion of phenolic compounds, because previous investigators have suggested that faecal excretion of phenolic compounds adequately reflected protein fermentation whereas urinary excretion did not (Birkett et al. Reference Birkett, Muir, Phillips, Jones and O'Dea1996).
A tendency towards a decrease in urinary excretion of both total p-cresol and labelled p-cresol was noted. Statistical significance was not obtained (Table 6). No increase in faecal excretion of p-cresol or labelled p-cresol was observed. Neither urinary nor faecal excretion of phenol was altered by inclusion of inulin into the meal. Labelled phenol was hardly ever recovered in faeces.
Table 6 Effect of long-term administration of inulin on the excretion of phenol and p-cresol in urine and faeces in seven subjects (Medians and interquartile ranges (IQR))
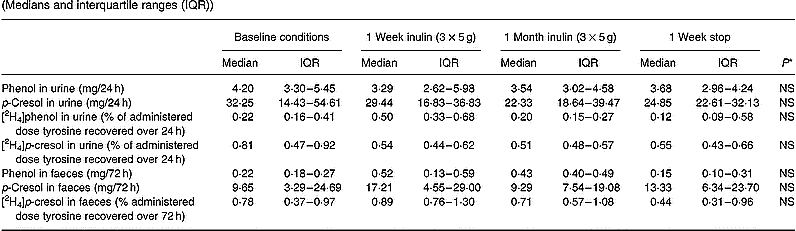
* Friedman ANOVA, α = 0·05.
Discussion
Colonic fermentation of proteins results in the formation of ammonia, nitrosamides, thiol and phenol compounds which are generally believed to be toxic to human metabolism.
Besides the characteristics of the bacterial flora and colonic transit time, the available fermentable carbohydrates:N ratio has been found to be an important factor influencing the fermentation process. Many in vitro studies have addressed several of the issues regarding protein and carbohydrate fermentation, by altering pH of faecal incubations or manipulating N or carbohydrate supply (Vince & Burridge, Reference Vince and Burridge1980; Vince et al. Reference Vince, McNeil, Wager and Wrong1990; Walker et al. Reference Walker, Duncan, McWilliam Leitch, Child and Flint2005). These studies allow us to look into specific effects of changes in the gut environment separately and have provided important information regarding SCFA production and the composition of bacterial flora, events that remain very difficult to study in vivo. Both in vitro and in vivo studies investigated the ability of fermentable carbohydrates to decrease the final concentration of products of protein fermentation. Fermentable carbohydrates are believed to influence protein fermentation both by increasing the bacterial uptake of intermediary metabolites of protein breakdown and by decreasing bacterial metabolism of proteins.
The former effect was often evaluated by measuring the faecal N excretion. As energy is largely derived from carbohydrate fermentation, it is believed that administration of fermentable carbohydrates stimulates bacterial growth and incorporation of ammonia into the bacteria, resulting in an increased faecal N excretion (Cummings et al. Reference Cummings, Hill, Bone, Branch and Jenkins1979; Vince et al. Reference Vince, McNeil, Wager and Wrong1990; Weber, Reference Weber1997). However, it is important to keep in mind that an increase in faecal N excretion is not necessarily due to an increased bacterial uptake of N, but can also be caused by certain physical properties of the non-digestible carbohydrate. For instance, some carbohydrates increase the viscosity of the intestinal contents in the small bowel, causing a decreased digestion of nutrients, including proteins, resulting in a higher amount of N reaching the colon and hence a higher faecal N excretion (Schneeman, Reference Schneeman1999; Vince et al. Reference Vince, McNeil, Wager and Wrong1990). Similarly, carbohydrates causing an acceleration of colonic transit will also lead to an increased faecal N excretion, without affecting protein fermentation (Stephen & Cummings, Reference Stephen and Cummings1980b; Weber, Reference Weber1997). Hence, it is important to carefully select the carbohydrate used to influence colonic fermentation. In the present study, inulin was chosen as the fermentable carbohydrate, because previous studies had shown that inulin does not influence digestion in the small bowel and does not accelerate colonic transit (Den Hond et al. Reference Den Hond, Geypens and Ghoos2000; Geboes et al. Reference Geboes, Luypaerts, Rutgeerts and Verbeke2003).
Ammonia in the colon originates from proteolysis as well as from ureolysis. In the present study, lactose [15N, 15N]ureide was used to evaluate the fate of ammonia. Upon oral administration, the molecular bond between the carbohydrate moiety and urea in LU has been shown to resist enzymic degradation in the human upper gastrointestinal tract (Wutzke et al. Reference Wutzke, Heine, Plath, Leitzmann, Radke, Mohr, Richter, Gulzow and Hobusch1997). When the labelled glycosyl ureide reaches the colon, it is degraded by bacterial enzymes to [15N, 15N]urea, which further undergoes rapid hydrolysis with the production of 15NH3. In this way, labelled LU is an efficient vehicle to deliver a known amount of labelled ammonia into the colon.
However, in the present results, a fraction (10 %) of the administered dose of 15N was consistently found in the 0–6 h urine collection, suggesting absorption of the marker in the small intestine. Other investigators have identified glucose [13C]ureide in urine upon oral administration of lactose [13C]ureide (Heine et al. Reference Heine, Berthold and Klein1995; Morrison et al. Reference Morrison, Dodson, Preston and Weaver2003). It is assumed that lactase, a brush-border enzyme in the small bowel, converts LU into glucose ureide which is absorbed in the small intestine and excreted in urine without further metabolism in the human body. Although the methods used in the present study did not allow identification of the chemical nature of the label in urine, we assume that most of the label recovered in the 0–6 h urine collection is present in the form of glucose [15N, 15N]ureide. As a consequence, the excretion of 15N in the 6–24 h urine collection is a more appropriate indicator of the absorption of ammonia in the large bowel.
The second substrate used consisted of egg proteins, intrinsically labelled with [2H4]tyrosine. Even in physiological conditions, some degree of malabsorption exists upon oral administration of egg proteins so that a small proportion is delivered to the colon and degraded by the bacterial flora (Evenepoel et al. Reference Evenepoel, Claus, Geypens, Hiele, Geboes, Rutgeerts and Ghoos1999). Bacterial metabolism of tyrosine results in the formation of phenol and p-cresol. These compounds are either excreted in faeces or absorbed through the colonic mucosa, detoxified by either glucuronide or sulfate conjugation in the colonic mucosa and the liver, and subsequently excreted in urine (Cummings et al. Reference Cummings, Hill, Bone, Branch and Jenkins1979). As a consequence, both urinary and faecal excretions reflect the production of phenolic compounds and hence the degree of bacterial fermentation of tyrosine.
The results of the present study indicate that inclusion of inulin in a meal causes other effects on protein handling in the colon than a dietary intervention for 4 weeks does.
In the first part of the present study, a single dose of inulin was administered together with the labelled substrates. In this case, it was expected that fermentation of the carbohydrate would provide energy to the bacteria, resulting in an increased uptake of ammonia. Simultaneously, bacterial fermentation of proteins may be influenced by changes in colonic pH, by catabolite repression and by an increased uptake of amino acids or intermediary metabolites (Vince et al. 1980; Cummings & Bingham, Reference Cummings and Bingham1987; Weber et al. Reference Weber, Banwell, Fresard and Cummings1987; MacFarlane et al. Reference MacFarlane, Gibson and Cummings1992). A significant increase in faecal 15N excretion, accompanied by a proportional decrease in urinary 15N excretion, was observed. Since no effect of inulin was observed on transit time, stool weight or faecal dry weight, it can be assumed that the variations in 15N excretion were not caused by an acceleration of colonic transit, but were due to alterations in the bacterial metabolism. This was confirmed by measurement of N and labelled N in the bacterial fraction of faeces. Reliable measurements of N and labelled N were obtained only when the volunteers had ingested inulin simultaneously with the labelled substrate. The content of N and labelled N in the bacterial pellets was below the limit of reliable measurements on every other occasion, including all the experiments in the long-term dietary intervention study. This is due to the small size of the bacterial fraction recovered in a 250 mg freeze-dried fresh faecal sample. However, the fact that reliable measurements were obtained whenever inulin was ingested together with the labelled marker indicates that an increased bacterial fixation of N occurs when carbohydrate is fermented.
Simultaneous administration of inulin with the test meal (single dose) also resulted in a statistically significant reduction of urinary [2H4]p-cresol excretion. Faecal recovery of labelled phenolic compounds was low, and the amount of labelled phenolic compounds present in the bacterial fraction would be below detection limits. Therefore, we cannot prove that the decrease in urinary [2H4]p-cresol is caused by an enhanced uptake of tyrosine for bacterial biosynthesis, a hypothesis that has been put forward by other authors (Cummings et al. Reference Cummings, Hill, Bone, Branch and Jenkins1979; Birkett et al. Reference Birkett, Muir, Phillips, Jones and O'Dea1996).
In the second part of the present study, the influence of a 4-week dietary intervention with inulin was evaluated. Inulin was never ingested on the morning of the test or administered together with the test meal, in order to exclude effects caused by fermentation of the carbohydrate. It was expected that the inclusion of inulin into the diet would cause a change in protein fermentation products through a modification of the composition of the colonic bacterial populations, accompanied by changes in microbial enzyme activity (Jenkins et al. Reference Jenkins, Kendall and Vuksan1999; Cummings et al. Reference Cummings, Macfarlane and Englyst2001; Roberfroid, Reference Roberfroid2001). Dietary intervention with inulin is known to selectively stimulate the growth of bifidobacteria (Gibson et al. Reference Gibson, Beatry, Wang and Cummings1995; Bouhnik et al. Reference Bouhnik, Flourie, Riottot, Bisetti, Gailing, Guibert, Bornet and Rambaud1996). In the present study, a mean increase in stool weight of 32 g/d was observed upon inclusion of 15 g inulin into the diet during 1 week, representing a value of approximately 2 g stool weight increase per g inulin consumed. These results confirmed previous studies investigating the effects of addition of inulin to the diet during 1 or 2 weeks (Gibson et al. Reference Gibson, Beatry, Wang and Cummings1995; Den Hond et al. Reference Den Hond, Geypens and Ghoos2000). However, this tendency towards an increase in faecal bulk was lost when inulin was taken for a longer period.
Previous investigators generally assumed that an increase in stool weight after stimulation of carbohydrate fermentation was caused by an increase in bacterial biomass (Gibson et al. Reference Gibson, Beatry, Wang and Cummings1995; Den Hond et al. Reference Den Hond, Geypens and Ghoos2000). However, it was shown that inclusion of inulin in the diet changed the relative proportions of bacterial species but did not cause an increase in total bacterial counts (Gibson et al. Reference Gibson, Beatry, Wang and Cummings1995; Bouhnik et al. Reference Bouhnik, Flourie, Riottot, Bisetti, Gailing, Guibert, Bornet and Rambaud1996). The results of our long-term dietary intervention study are in agreement with these observations, since we did not observe increased faecal excretion of either total or labelled N, which would reflect bacterial uptake of ammonia and hence bacterial growth.
A tendency towards a decreased urinary excretion of both labelled and unlabelled p-cresol was noted in the present study, which may reflect a change in microbial activity. However, statistical significance was not obtained. Once again, the effect was most clear after inclusion of inulin into the diet during 1 week.
In conclusion, the results of the present study indicate that lactose [15N, 15N]ureide is an appropriate tool to investigate the fate of colonic ammonia. Egg proteins, intrinsically labelled with [2H4]tyrosine, may provide an appropriate tool to study changes in the production of protein fermentation metabolites. However, the low overall recovery of the label is an important drawback. Using these two substrates, we were able to show that inulin influences the colonic metabolism of dietary protein and N it is ingested with. On the other hand, systematic intake of inulin did not induce changes in the bacterial metabolism of protein. Further studies that measure both bacterial populations and excretion of the labelled markers are currently being performed.
Acknowledgements
The present study was carried out with financial support from the Commission of the European Communities, specific RTD programme ‘Quality of Life and Management of Living Resources’, QLK1-2001-00 431 ‘Stable isotope applications to monitor starch digestion and fermentation for the development of functional foods’. It does not necessarily reflect its views and in no way anticipates the Commission's future policy in this area.










