INTRODUCTION
Noroviruses, formerly known as ‘Norwalk-like viruses’ and ‘small, round-structured viruses’, are a major cause of gastroenteritis worldwide [Reference Barker, Vipond and Bloomfield1, Reference Johnston2]. They are also the most common cause of gastrointestinal disease in the USA, with an estimated 23 million cases occurring annually [Reference Johnston2, Reference Cooper and Blamey3]. Norovirus gastroenteritis (NVG) spreads rapidly in environments such as hospitals, nursing homes, day-care centres, residential and domestic homes, schools, hotels and cruise ships [Reference Barker, Vipond and Bloomfield1, Reference Fankhauser4]. NVG outbreaks also frequently occur at recreational activities and events with catered meals [Reference Sartorius5]. Based on available survey information, norovirus is by far the most common cause of outbreaks of non-bacterial infectious intestinal disease. In parallel with the development of novel, more sensitive detection methods, the association of norovirus with gastroenteritis outbreaks is well documented. The most common settings were in healthcare facilities, accounting for 91% of reported outbreaks in The Netherlands, 96% in the USA and 79% in England and Wales, respectively [Reference Vinje, Altena and Koopmans6–Reference Lopman8]. Norovirus outbreaks are particularly difficult to control due to their stability in the environment and efficient transmission by person-to-person contact [Reference Johnston2, Reference Lopman8–10]. They have significant economic implications for healthcare institutions because of considerable knock-on effects on care provision through ward closures and staff sickness [Reference Johnston2, Reference Radford, Gaskell and Hart9, Reference Caul11].
From January 2005 to April 2007, a total of four episodes of NVG outbreaks occurred in a psychiatric care centre situated in one separate building of a regional teaching hospital in Taiwan. This study aimed to identify the epidemiological characteristics of each single outbreak, and differentiate epidemiological links between the different outbreaks by using epidemiological data and molecular tools to detect norovirus strains. The identification of reasons for the outbreaks was also an objective of this study. In addition, we summarize the infection control and prevention measures which were used to contain the norovirus outbreaks within this institution and evaluate the outcome of these measures.
METHODS
Clinical setting
The Wei-Gong Memorial Hospital (WGMH) is a 979-bed regional teaching hospital in Miaoli County, Taiwan. There are three separate buildings which provide acute, chronic and psychiatric care. This hospital also has an affiliated psychiatric nursing home (PNH) located in a different building from the psychiatric care centre. The Dongsing branch of the psychiatric care centre is located in one separate building which is 5 km away from the other buildings. It is a seven-floor building and consists of six wards (wards 1, 2, 3, 5, 7, 8). Each of these wards is located on one floor. All of the wards are arbitrarily classified into three categories for hospitalized care-giving: (1) acute psychosis in wards 2 and 3, (2) chronic psychosis in wards 1, 7, 8, and (3) mixed care in ward 5. The psychiatric care centre contains 445 beds including 126 beds for acute psychiatric care, 259 for chronic care, and 60 for day care. The centre admits adult psychiatric patients aged >15 years. All of the NVG patients were hospitalized within the psychiatric care centre and did not have direct communication with their families or friends during each outbreak, except for five PNH residents and seven healthcare workers (HCWs).
Case definition
A case was defined as a patient who had acute onset of diarrhoea (⩾2 loose stools in a 24-h period), vomiting (any episode of emesis), an unexplained abdominal pain or fever (body temperature ⩾37·8°C) and an average duration of symptoms of 12-60 h during the outbreaks. The duration of illness was defined as the number of days between the first and final dates of symptoms. An outbreak was defined as ⩾3 cases in the psychiatric care centre with onset dates within 7 days of each other [Reference Johnston2, Reference Green7, Reference Lopman8, Reference Rockx12]. Although a serological or RT–PCR positive for diagnosis of norovirus infection is usually essential, cases were considered to be norovirus positive if stool samples from more than two patients in each outbreak were positive with norovirus-specific RT–PCR tests [Reference Johnston2]. Because norovirus was the most likely pathogen according to the patients' epidemiological setting (in place and time) and no other pathogens were detected from bacterial cultures, patients who were norovirus negative but had symptoms of vomiting or diarrhoea were also included. In addition, all of the patients from one of the psychiatric wards who were affected with an acute onset of symptoms characteristic of NVG were also included as cases. Patients with illness were excluded if the symptoms were due to ingestion of laxative drugs or other medical diseases such as incontinence. An outbreak was presumed to be over when no new case occurred for more than 2 weeks.
Risk factor analysis and attack rates
The following potential risk factors were analysed: age, gender and underlying psychiatric disorder (by ward categories). The NVG attack rates of hospitalized patients in each ward category, hospital staff and PNH residents were calculated by dividing the number of cases by the total number of hospitalized patients. Repeated infections were also included in the analysis and were defined as a patient who experienced ⩾2 infections during different outbreaks.
Laboratory diagnosis
Randomly selected stool samples of affected patients were sent to the Centers for Disease Control in Taiwan for norovirus testing during the investigation of each outbreak. Regarding transmission evidence, stool samples from asymptomatic HCWs who had close contact with NVG patients, environmental surfaces and toilet facilities in one affected ward were also sent to the reference laboratory during the second outbreak in 2006 and third outbreak in early 2007. Viral RNA extraction and RT–PCR using the primers G1SKF and G1SKR for norovirus genogroup I (GI) and G2SKF and G2SKR for genogroup II (GII) were performed as described previously [Reference Wu13]. All norovirus RT–PCR products were further determined by sequencing and phylogenetic analysis. Cultures for enteropathogenic bacteria, including Salmonella, Shigella, Vibrio, Escherichia coli O157, and Clostridium difficile from stool samples were done at the microbiological laboratory of WGMH.
Management of outbreaks and evaluation of control measures
Multiple infection-control measures were implemented to interrupt the transmission of infection and prevent spread to other unaffected wards as soon as a cluster of gastroenteritis cases was reported by nursing staff. A cohort programme was the first measure. The affected patients were confined to their rooms and positioned in a side area of the ward. A cordon was established to demarcate contaminated and clean areas. Standard precautions including wearing gowns, masks, gloves, head caps and shoe caps were universally implemented when HCWs entered the contaminated areas. All staff in the affected wards were asked not move to unaffected wards. The head nurses were also restricted from moving between different wards. Confinement of new admissions to the detention ward for at least 5 days and group therapy sessions were halted during the outbreak period. Environmental-service personnel and their equipment were cohorted in the affected areas and movement between affected and unaffected areas was restricted. Vomitus and faecal spillage were soaked with 0·5% (5000 ppm) bleach for at least 30 min and then flushed into the sanitary sewer.
Hand hygiene practices included (1) periodic broadcasting reminding unaffected patients to wash hands hourly, (2) HCWs carried a 75% alcohol solution to help unaffected patients with hand washing and to disinfect door handles during work, (3) alcohol solution was made available to visitors and a poster on hand washing to improve adherence to hand hygiene was placed at the entrance of wards, and (4) active spraying of alcohol solution on the hands of visitors by a guard at the entrance of the building. In addition, HCWs were regularly encouraged to perform precise washing of hands with soap, chlorhexidine, and water after completing work and before meals.
Appropriate environment-cleaning measures included: (1) stabilized patients instructed to clean and disinfect the beds, windows, and chairs of their private rooms with 0·05% bleach daily, (2) floors of the communal areas were cleaned with freshly prepared bleach at least once daily by environmental-service personnel, (3) tables, telephones, computer keyboards, chart envelopes, door handles and other frequently touched surfaces of nursing stations were wiped with 0·05% bleach every 8 h, and (4) a door mat rinsed with 0·5% bleach was provided at nursing stations, stairs, and wards and replaced every 8 h.
The education programme included (1) reinforcement and monitoring of infection-control practices during daily surveillance rounds by infection-control professionals, (2) confirmation that the correct preparation and effective concentration of fresh bleach was used, (3) demonstration of adequate wearing of personal protective equipment (PPE) and procedures for hand hygiene, especially for environmental-service personnel, cooks, and laundry workers were adhered to, and (4) patient's clothes were soaked with 0·05% bleach for 30 min before cleaning and those of affected areas were cleaned last.
Other important instructions included: (1) restrictions placed on visiting staff and related HCWs to reduce the frequency and number of staff in daily ward rounds with visiting and leaving times strictly recorded, (2) because of limited staff resources during off-peak times and holidays, garbage in affected areas was promptly sent to the entrance of wards by duty nurses and then removed from the premises by environmental-service personnel, (3) symptomatic or norovirus-positive HCWs were placed on sick leave for at least 72 h after their last symptoms, and (4) cessation of occupational therapies or similar activities was implemented in affected wards, including unaffected patients, for at least 14 days after the last symptomatic case unless in medical need.
Regarding observation of implemented control measures and assessment of outcome, each infection-control professional was asked to witness and take photographs of the detailed measures including preparation of effective bleach daily. The records for caring for patients and handing over to the next shift of the infection-control professional were cross-checked completely.
Statistical analysis
All data were expressed as mean (standard deviation). Spearman's rank correlation coefficient was used to analyse the possible correlation between two ordinal variables of age, presenting symptoms and duration of illness. This coefficient measures consistency. If two variables are consistently related, their ranks will be linearly related. The possible range of this coefficient is from −1 to +1.
RESULTS
Between January 2005 and April 2007, a total of four norovirus outbreaks occurred within this psychiatric care centre. The periods were: outbreak 1 (5 January–23 January 2005), outbreak 2 (30 January–28 February 2006), outbreak 3 (28 December 2006–24 January 2007) and outbreak 4 (13 April–27 April 2007). The number of affected patients was: outbreak 1 (n=82), outbreak 2 (n=31), outbreak 3 (n=58) and outbreak 4 (n=13). In comparison with the previous three norovirus outbreaks, outbreak 4 was terminated quickly. The characteristics of these patients are summarized (Table 1).
Table 1. The characteristics of patients in four norovirus outbreaks during 2005–2007
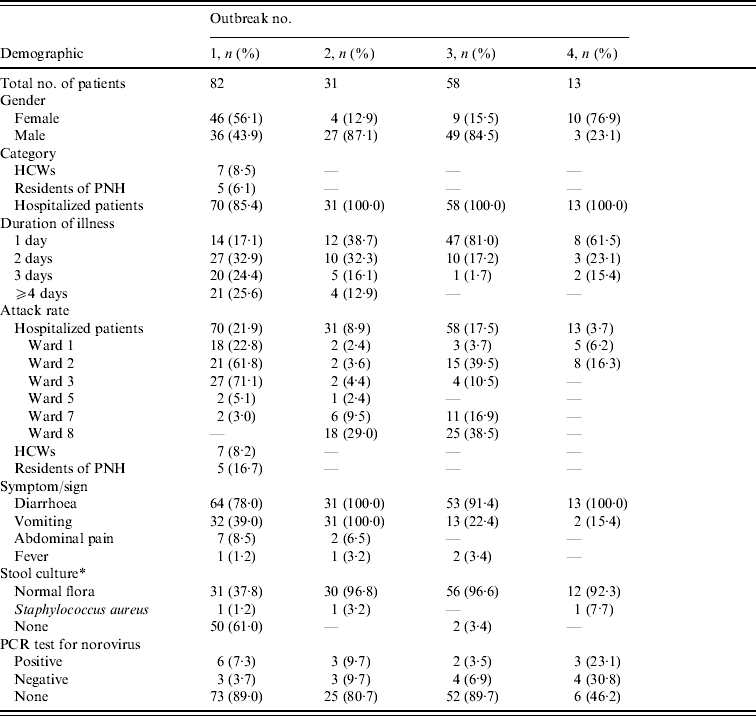
HCW, Healthcare worker; PNH, psychiatric nursing home.
* Stool culture: for enteropathogenic bacteria including Salmonella, Shigella, Vibro, Escherichia coli O157 and Clostridium difficile.
Time trends and seasonality
The epidemic curve of all four outbreaks from January 2005 to April 2007 is plotted in Figure 1. The major seasonal peaks were between late winter and early spring, during January and February, which is the coldest time of the year on this island. However, a major peak was also noted in the last outbreak in April 2007.
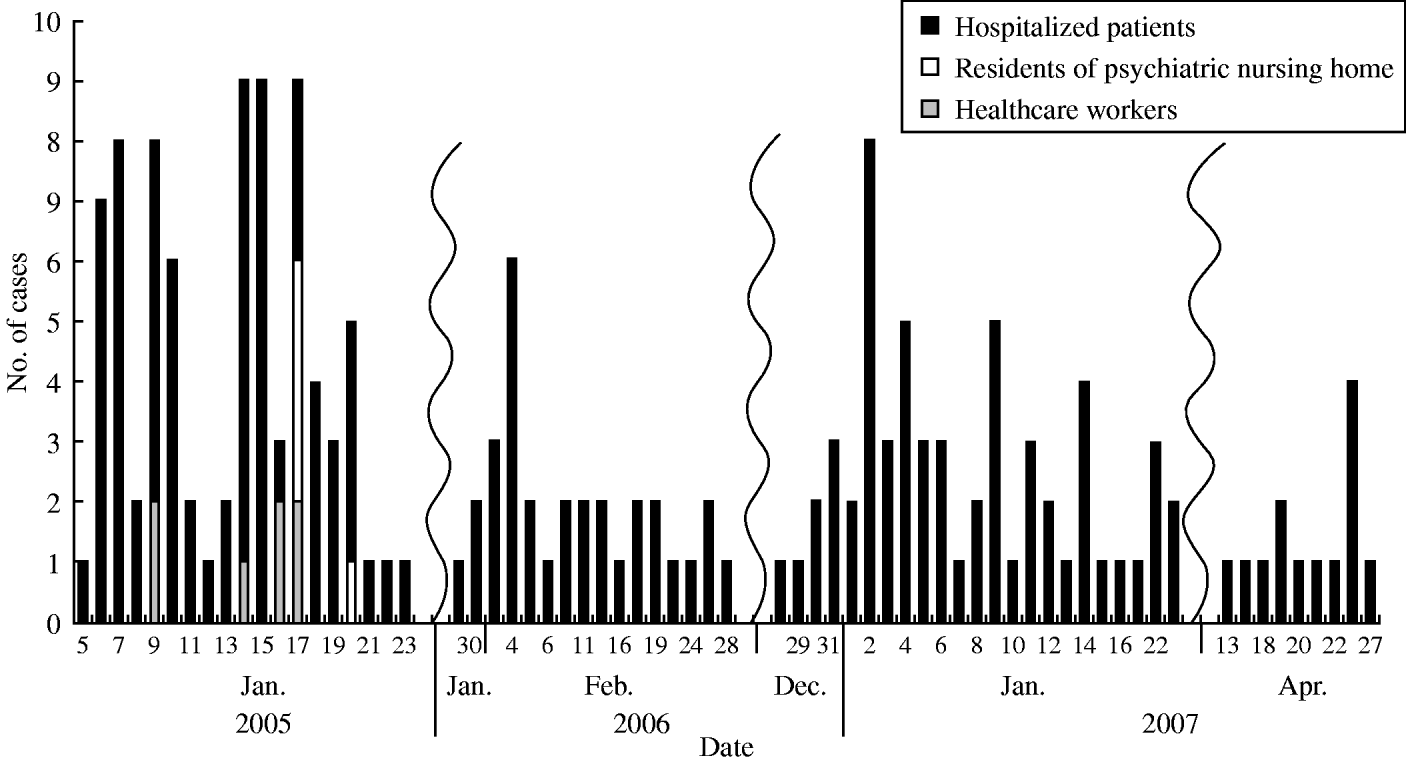
Fig. 1. Epicurve of illness onset of all affected patients in the four norovirus outbreaks between 2005 and 2007 (n=184).
Age distribution
The age distribution of all affected patients in the four outbreaks which occurred from January 2005 to April 2007 is illustrated in Figure 2. The mean age was 46.4±3.2 years. The attack rates by age showed an inverted U-shaped pattern, with the higher rates in patients aged between 30 and 59 years. The numbers of affected patients aged 30–39, 40–49 and 50–59 years were 45 (24·5%), 51 (27·7%) and 38 (20·7%), respectively.
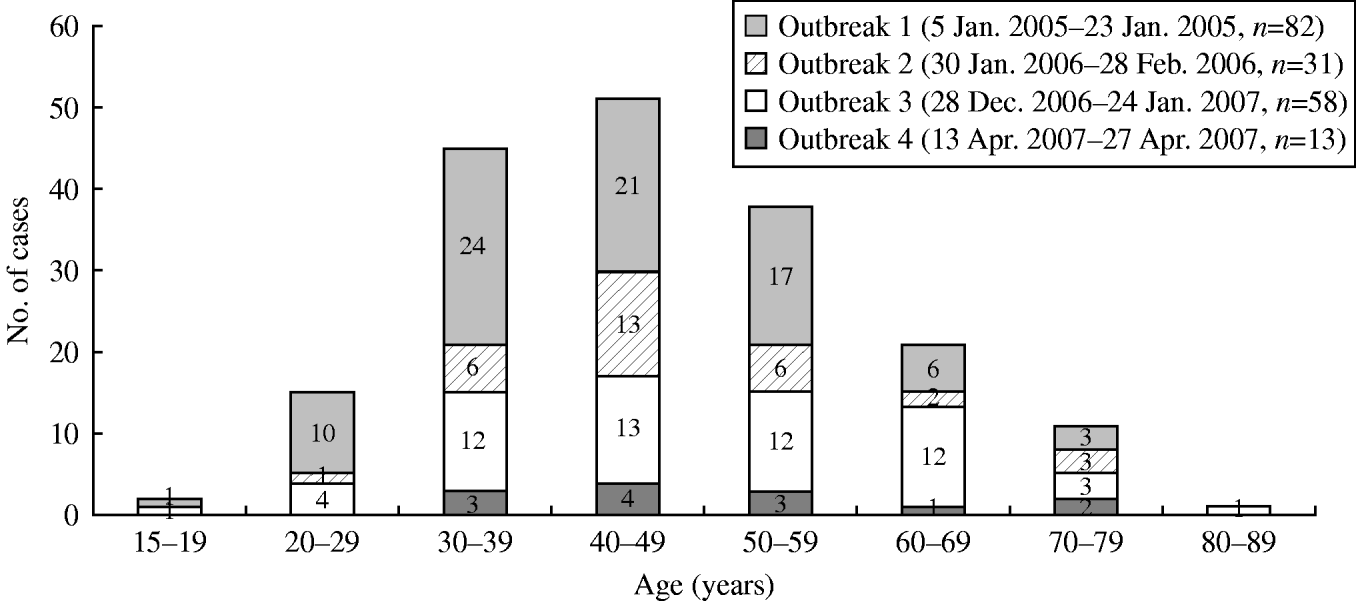
Fig. 2. Age distribution of patients with norovirus gastroenteritis in four outbreaks between 2005 and 2007.
Analysis of clinical manifestations
There were 184 patients with NVG during the period of the four outbreaks. Seven HCWs and five PNH residents were affected in outbreak 1, whereas no HCW or PNH resident developed NVG in the other three outbreaks. The five PNH residents who lived in one separate building were confined to their rooms during the time of this outbreak. Although we precisely reviewed all possible factors related to spread, no prominent contributory factor linking transmission between these two buildings was detected. The seven HCWs included five nurses, one attendant and one rehabilitation therapist. The mean age was 43 years in outbreak 1, 48·3 years in outbreak 2, 49 years in outbreak 3 and 50·9 years in outbreak 4. The male:female ratio in each outbreak was 0·78, 6·75, 5·44, and 0·30, respectively.
Symptoms and duration of illness
The most common symptom was diarrhoea (161/184, 87·5%), followed by vomiting (47/184, 25·5%), abdominal pain (9/184, 4·9%) and fever (4/184, 2·2%) respectively. Further analysis of the distribution of defined symptoms by age group revealed that the majority of patients who developed diarrhoea were young and middle-aged adults. However, the presentation of the other three defined symptoms was not significantly associated with any age category.
The mean duration of all 184 affected patients was 2·1±1·5 days. Most patients (159/184, 86·4%) experienced illness for 1–3 days. Each mean duration of NVG symptoms of different outbreaks was 2·8 days (range 1–6 days), 2·4 days (1–13 days), 1·2 days (1–3 days) and 1·5 days (1–3 days), respectively. The average duration of illness in outbreaks 3 and 4 in 2007 was shorter than in outbreak 1 in 2005 and outbreak 2 in 2006. The mean illness duration was 3·5 days (1–6 days) in patients aged 15–19 years which was longer than those in the elderly. In addition, we also found that the mean duration in cases with acute psychosis was longer than those with chronic psychosis.
Multivariate analysis including age, presenting symptoms, and duration of illness revealed three significant correlations (Table 2). A positive correlation between duration of illness and frequency of abdominal pain highlighted a higher incidence of abdominal pain associated with longer duration of illness. A negative correlation between frequency of abdominal pain and age implied that younger age was correlated with a higher incidence of abdominal pain. Furthermore, a negative correlation between the frequency of vomiting and diarrhoea demonstrated a higher occurrence of diarrhoea associated with a lower incidence of vomiting.
Table 2. Multivariate analysis of clinical manifestation of all patients in four norovirus outbreaks during 2005–2007 (n=184)
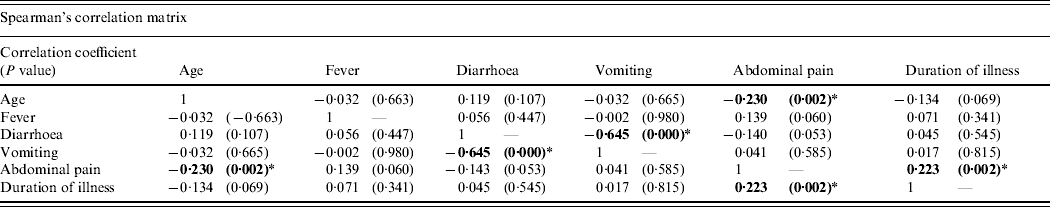
* Correlation is significant at the 0·01 level (two-tailed).
Ward distribution and attack rate of NVG
The attack rates of nurses, attendant, rehabilitation therapist and PNH resident in outbreak 1 were 7·9% (5/63), 5·6% (1/18), 25·0% (1/4) and 16·7% (5/30), respectively. After exclusion of seven HCWs and five PNH residents, a total of 172 hospitalized patients had an episode of NVG. The mean attack rate of NVG was 12·7% (172/1351). The attack rate of each single outbreak is shown in Table 1. The numbers of affected patients in the acute, chronic and mixed wards were 79 (45·9%), 90 (52·3%) and three (1·7%). The NVG attack rates were 22·9% (79/345), 10·7% (90/839) and 1·8% (3/167), respectively.
Repeated infections
Reviewing data for 184 patients between 2005 and 2007 revealed that 17 had experienced recurrent NVG during the four outbreaks. Sixteen patients had two episodes of NVG during different outbreaks. The remaining patient had three episodes of NVG which occurred during outbreaks 1, 3 and 4. The overall re-infection rate was 10·2% (17/166).
Microbiological findings
Stool samples from 71·7% (132/184) of affected patients were sent for bacterial culture and 97% yielded growth of normal flora. A total of 28 stool specimens collected from patients in these four outbreaks were further screened for norovirus by ELISA method and RT–PCR. The number of stool samples sent to the Centers for Disease Control in Taiwan for norovirus confirmatory tests of each outbreak was 9, 6, 6 and 7, respectively. The results of ELISA method and RT–PCR for these samples were consistent. Fourteen (50%) samples were positive, which comprised ⩾2 norovirus-positive cases in each outbreak (6, 3, 2, 3 positive cases in different outbreaks). RT–PCR data showed that three of these outbreaks were caused by GII and one was caused by GI norovirus (Fig. 3). We found that all GII norovirus sequences belonged to GII.4 and GI belonged to GI.14 and they were identical within outbreaks. Virologically, a total of three different clones of norovirus were found.
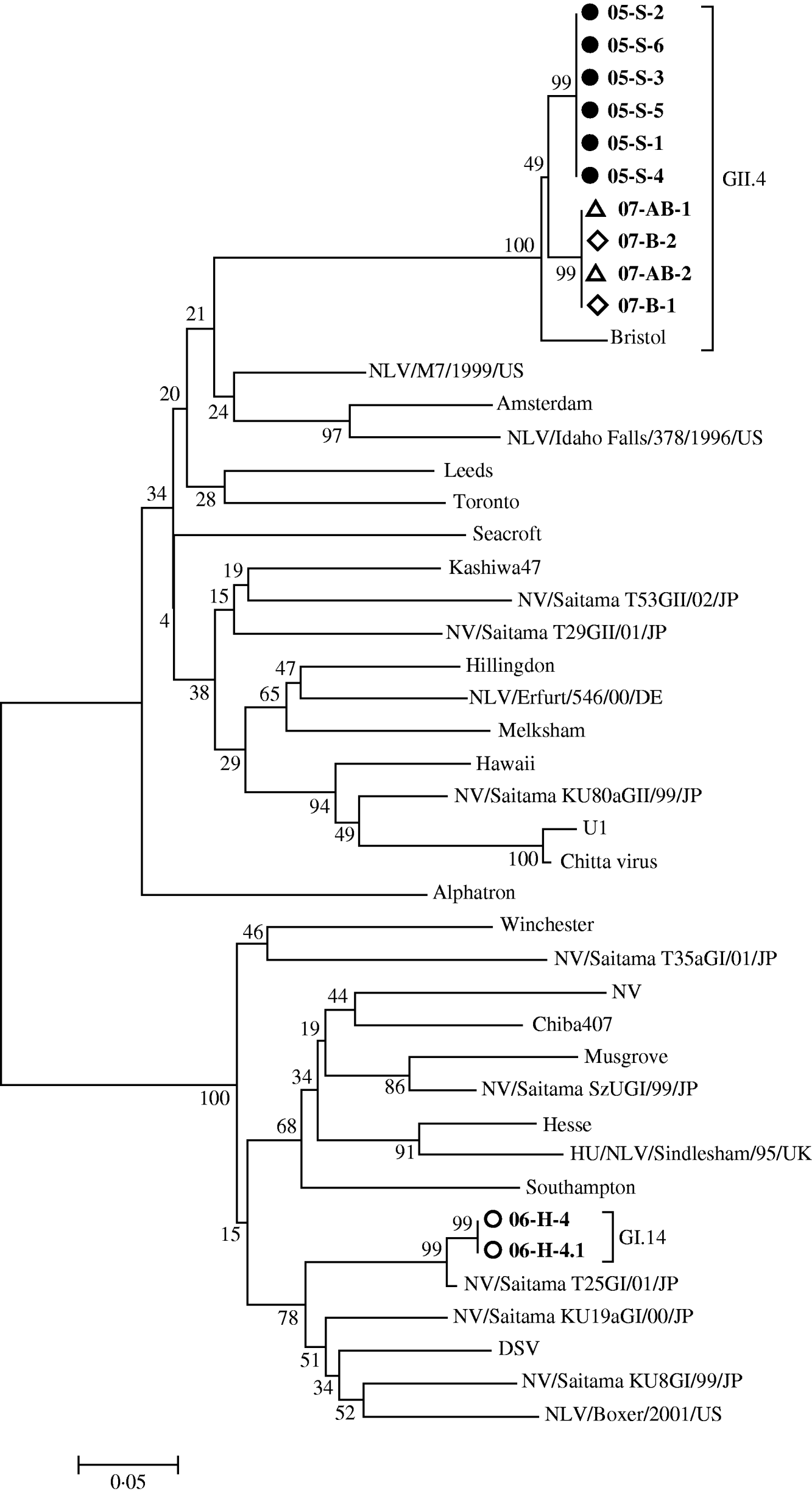
Fig. 3. Phylogenetic tree of the norovirus sequences detected in this study (represented in bold). Norovirus nucleotide sequences were constructed with the partial N-terminal capsid region [Reference Wu13]. We gave each norovirus RT–PCR-positive case a sequence distinct name in the order of outbreak year. The same symbol in front of the sequence name means the same outbreak, • represents outbreak 1, ○ represents outbreak 2, ◊ represents outbreak 3 and ▵ represents outbreak 4. The numbers on the branches indicate the bootstrap values for the clusters. Bootstrap values of ⩾95 were considered statistically significant for the grouping.
During the investigation of outbreak 2 in 2006, norovirus tests for stool sample were performed from five randomly selected HCWs who had close contact with hospitalized patients and were considered to have asymptomatic infections. Twenty percent (1/5) were norovirus positive. The result was identical to that of NVG patients. Six samples of environmental surfaces and water from a private room of affected patients in ward 8, including tap-water of the restroom, cover and flush-button of toilet, outlets of a hydrant and water-supply machine, and door handles as recommended by the Centers for Disease Control in Taiwan were sent for detection of norovirus. None of these samples were positive. Similarly, ten stool samples of asymptomatic HCWs with a high probability of spreading norovirus were sent for detection during the period of outbreak 3. All of these samples were tested negative.
DISCUSSION
Noroviruses can infect individuals in any age group. The recalcitrant behaviour of norovirus outbreaks is attributable to the characteristics of a low infectious dose (<102 viral particles), relative stability in the environment, spread via multiple modes of transmission, prolonged asymptomatic shedding and lack of lasting immunity. Previous studies demonstrated that both airborne transmission via aerosolized droplets of vomitus within a defined airspace and contact with environmental surfaces via widespread fomite contamination in confined spaces can prolong the course of an outbreak [Reference Marks14–Reference Jones16]. These transmission modes facilitate outbreaks and make them difficult to control [17, Reference Weber18]. Several reports describing outbreaks of NVG in semi-closed institutions, such as hospitals and long-term-care facilities, usually have a unique picture, including (1) a winter peak, (2) significantly higher death rates, (3) prolonged duration, (4) smaller size, (5) less likely to be foodborne, and (6) rapid spread [Reference Lopman8, Reference Wu19]. Psychiatric settings place more restrictions on the behaviour of patients and have more prolonged levels of contact between patients. These features may explain why the duration of outbreaks at these institutions are usually more prolonged. This study demonstrated characteristics of four consecutive NVG outbreaks within 3 years and highlighted the infection-control measures required to terminate periodic occurrences of outbreaks in a psychiatric centre.
Compared to outbreaks in paediatric patients, who are more prone than adults to develop vomiting and have a longer duration of illness (which has been reported to be as long as 140 days illness in compromised children) [10, Reference Simon20, Reference Cheng21], the patients in these four consecutive outbreaks in our psychiatric care centre had a shorter duration of illness and a lower incidence of vomiting. Previous studies found that recovery was slowest in the oldest age group and that highest incidence of infection occurred in paediatric and geriatric populations [Reference Mattner22, Reference Lopman23]. Illness durations of 12–60 h and ⩽2 days were reported by other investigators [10, Reference Rockx12, Reference Lopman23, Reference Bresee24]. However, our study demonstrates that younger patients had longer duration.
Due to their occurrence in winter months in the majority of reports, norovirus outbreaks have been referred to as ‘winter vomiting disease’ [Reference Lopman8, 24–Reference Hart, Radford, Fuchs and Podda26]. The four outbreaks in the current study all had peaks in late winter and early spring. Outbreak 1 exhibited two peaks and affected the most psychiatric patients. This may have been attributable to lack of experience in recognition and management of a NVG outbreak. The current study found that hospitalized acute psychiatric patients had higher susceptibility to norovirus infection than chronic psychiatric patients. This may be due to more severe underlying psychiatric diseases and more frequent abnormal behavioural activities in acute psychiatric patients. In our study, 17 affected patients experienced more than two episodes of infection during the 3-year study period encompassing the four outbreaks, including three patients who experienced episodes of NVG during outbreaks 3 and 4 which were caused by the same norovirus clone. This finding highlights the lack of lasting immunity and is compatible with a previous hypothesis that susceptibility to norovirus infection is genetically determined [Reference Bresee24].
Patients in psychiatric care facilities had a significantly higher vulnerability to infection and greater opportunity for transmission of NVG [Reference Johnston2]. Although many studies demonstrated management strategies for terminating norovirus outbreaks, the control of outbreaks in psychiatric units presents unique challenges, mainly because of the behavioural impact of underlying diseases and the use of frequent group activities for therapy. In the current study, the mean outbreak duration was 23 days (range 15–30 days), which is longer than reported in previous studies [Reference Lopman8, 17]. Early detection of NVG is difficult and outbreaks can occur rapidly. Although multiple infection-control measures were implemented from 2005 and 2006, the outbreaks continued in our psychiatric centre. In 2007, more stringent infection-control interventions were reinforced including interruption of infective sources and prevention of spread to other unaffected wards as has been previously recommended [Reference Johnston2, Reference Chadwick25]. The shorter duration of outbreak 4 in April 2007 than in the previous three outbreaks may be attributable to these interventions.
Due to the unfortunate consequences of periodic and difficult to control norovirus outbreaks, our infection-control measures were sweepingly reviewed and the following contributory problems were found: (1) there was no explicit, organized norovirus outbreak control guidelines for HCWs and hospitalized patients, (2) infection-control measures, provided by the infection-control professionals, could not be implemented completely, (3) delay in or not informing infection-control personnel about the occurrence of any unexpected vomiting or diarrhoea in patients or staff, or clustering cases of gastroenteritis, (4) movement of staff and HCWs, especially environmental-service personnel, was not prohibited during the outbreak periods, (5) inadequate disinfection due to insufficient concentration of bleach (500 ppm), and (6) incorrect methods of hand hygiene, especially waterless hand washing and shorter friction time with alcohol solution. For inactivation of feline caliciviruses, a surrogate of norovirus, alcohol solution has limited effect and requires longer contact time ranging from 30 s to 10 min [Reference Weber18, Reference Gehrke, Steinmann and Goroncy-Bermes27, Reference Sattar28]. Hand hygiene with alcohol-based hand-rubs was effective for noroviral disinfection and recommended in previous studies. Correct hand washing with liquid soap and water for 1 min, rinsing for 20 s has been advocated [Reference Johnston2, 29–Reference Said, Perl and Sears31]. For environmental disinfection, domestic bleach with a concentration of ⩾0·1% (1000 ppm) is necessary. Cleansing with a combined hypochlorite detergent formulation to decontaminate faecally contaminated environmental surfaces has also been emphasized [Reference Weber18]. From May to June 2007, infection-control measures were analysed and, following frequent communication between psychiatric HCWs and infection-control professionals, a practical comprehensive and responsive standard operating procedure (SOP) for management of infectious gastroenteritis in the psychiatric care centre was established. This SOP, a revision of recommendations from CDC of Taiwan, precisely described a multilateral infection control strategy and daily prevention measures to be implemented during the epidemic seasons, i.e. from November to April. The following interventions were made as part of this SOP: cohorted patients were moved from the ordinary ward to the drug-addiction ward (i.e. switched to an isolation ward); areas of detention rooms for newly admitted patients were established within each ward (a moratorium on discharges of hospitalized patients was simultaneously instituted); hand washing with soap and water or waterless alcohol-based hand-rubs with a higher alcohol content solution (90%) was emphasized [Reference Weber18, Reference Gehrke, Steinmann and Goroncy-Bermes27, 29, Reference Kampf, Grotheer and Steinmann30]; efficient environmental cleansing with ⩾0·1% (1000 ppm) hypochlorite was instituted [Reference Johnston2, Reference Cheng21, Reference Sattar28, Reference Doultree32]; and psychiatrists were reminded that evaluation of every patient with acute gastroenteritis was very important. After implementation of the SOP and regular surveillance, no further NVG outbreak was found.
Previous studies found that GII.4 was by far the most prevalent strain in outbreaks and that outbreaks were often dominated by one strain [Reference Said, Perl and Sears31, Reference Patel33]. GII.4 strain was also the dominant cause of previous NVG outbreaks in Taiwan [Reference Wu13]. In the current study, GII.4 noroviruses were confirmed to be the major aetiological strains of outbreaks. The presence of a single strain of virus in each outbreak also confirmed a common source of infection. This phenomenon is in accord with a report by Said et al. [Reference Said, Perl and Sears31]. Because of limitations in the virological analysis in our study, multiple introductions of NVG from the community could not be excluded. A stool sample from one of the 14 norovirus-positive cases in the current study that was sent to the reference laboratory on day 7 of illness also met the established description of 2–3 weeks duration of virus shedding.
The HCW who had a positive norovirus test during the investigation of outbreak 2 in 2006 was a member of staff of ward 7. This staff member had taken responsibilities and moved between wards 7 and 8 to cover nursing services during the outbreak period. As fresh faecally contaminated water is a possible source of noroviral infection [Reference Sartorius5], analysis of water by RT–PCR to support epidemiological evidence was performed in the reference laboratory. Nevertheless, none of the water samples tested positive for norovirus. These results indicate the linkage of the affected patients and asymptomatic HCWs in outbreak 2 and suggest that HCW contact (asymptomatic infection) played an important role in prolonging the duration of the outbreak.
There are several limitations in this retrospective study. The impacts of NVG outbreaks on psychiatric care centres can not be precisely estimated. Similarly to other studies, the chart records were incomplete. Detailed characteristics of affected patients were unavailable. The resources for norovirus confirmation tests were limited in the reference laboratory, and most affected patients were clinically and epidemiologically suspected cases. In addition, the results of norovirus tests from samples of environmental surfaces and water were all negative. The identification of reasons for outbreaks is difficult.
Psychiatric care centres are long-term-care facilities in which efforts to control outbreaks are usually hindered by the inability to detect them sufficiently early enough to confine the index patient due to behaviour associated with psychiatric disorders. A high index of suspicion for NVG should always be maintained during the winter season. The need for aggressive management of NVG in order to prevent rapid spread due to person-to-person contact or the faecal–oral route is important. Strict implementation of a cleaning and disinfection programme for the prevention, containment, and termination of norovirus outbreaks is mandatory. In conclusion, this study shows that early recognition of suspected cases by ward staff, rapid informing of infection-control professionals, aggressive interventions with an efficient cleaning and disinfection programme including the use of hand-rubs and observation of detailed preparation of effective bleach, daily cross-check of records for caring of patients and handing over to the next shift of infection-control professionals, and appropriate management of patients to shorten the duration of illness can be effective to stop and prevent norovirus outbreaks in psychiatric centres.
ACKNOWLEDGEMENTS
We thank Yu-Ting Li for data retrieval and entry, and Fang-Chin Liang for assistance in data collection and compilation of statistics.
DECLARATION OF INTEREST
None.









