Introduction
Dementia encompasses multiple sub-types and is a cause of significant disability that includes loss of independence, lower quality of life and significant caregiver burden.Reference Hogan, Jetté and Fiest 1 - Reference Fiest, Roberts and Maxwell 3
As the prevalence of dementia continues to increase, so does the number of people presenting to their primary care physicians with memory complaints.Reference Cutler 4 , Reference Kosteniuk, Morgan and O’Connell 5 This seems to represent an overall improvement in society’s awareness of dementia, but with it comes an increased burden on primary care and consequently on the specialists who see these patients.Reference Menon and Larner 6 The diagnosis of dementia remains clinical, as no single imaging or laboratory test is necessarily diagnostic of the condition. Therefore, many cases of subjective memory concerns (SMC) are referred on to specialists for assessment. Dementia is a common worry among the aging population.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 With people increasingly aware of dementia, a new trend has been developing in memory clinics: the “worried well”.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 This refers to patients who are worried they have dementia, but are in fact neurologically normal, and have a neuropsychological profile within normal limits for age and other demographic factors.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 It has been suggested that these patients often have friends, family or other associates with dementia.Reference Kinzer and Suhr 8
Memory concerns from patients are subjective, and may be influenced by psychological and environmental factors, such as exposure to someone with dementia.Reference Kinzer and Suhr 8 There have been conflicting data on the validity of subjective memory complaints from patients and the correlation with the patient’s true cognitive function.Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 , Reference Jessen, Wolfsgruber and Wiese 9 Subjective cognitive impairment (SCI) is common, and it has been suggested that it may be a prognostic indicator.Reference Reisberg and Gauthier 10 Some have considered SCI the beginning of the Alzheimer’s disease spectrum, and others question its validity due to inconsistent evaluation and definition.Reference Reisberg and Gauthier 10 However, SCI has been identified as a risk factor for mild cognitive impairment (MCI).Reference Reisberg and Gauthier 10 Many memory clinic patients present with SCI and these patients need to be properly evaluated and followed so as to provide support and close monitoring of changes in memory function.Reference Reisberg and Gauthier 10 The Self-Rating of Memory Score is a well-validated tool used to evaluate patients’ perceived memory concerns, and may therefore provide insight into SCI.Reference Squire and Zouzounis 11 Another screening tool used to evaluate cognitive function is the Mini-Mental Status Examination (MMSE), which has long been used and studied as a cognitive screen.Reference Mitchell 12 Repeat MMSE scores can be an important part of evaluating trends in cognitive ability over time, and has been validated using Telehealth and in person.Reference Mitchell 12 , Reference McEachern, Kirk, Morgan, Crossley and Henry 13 In contrast to screening, neuropsychological testing is an in-depth, standardized assessment of a multitude of cognitive domains with appropriate normative comparisons to facilitate interpretation. Ideally, dementia diagnoses (referred to as major and mild neurocognitive disorders in the new Diagnostic and Statistical Manual of Mental Disorders-V) includes neuropsychological assessment of simple and complex attention, speed of mental processing, language, visuospatial functioning, social cognition, executive function (behavior regulation, planning/organization, sequencing, and inhibition), and memory (encoding, consolidation, and retrieval). 14 Longitudinal neuropsychological testing allows for comparison of a person’s performance not only with the average obtained from normative data, but also change within an individual. Aside from SCI, other risk factors for dementia may include age, lower education levels, sleep concerns, and psychiatric illness.Reference Ngandu, von Strauss and Helkala 15 - Reference Modrego and Ferrández 18 A study by Kosteniuk et al.Reference Kosteniuk, Morgan and O’Connell 5 in 2015 found that the incidence of dementia increased from 2.8 to 5.1 and the prevalence rate increased from 2.6 to 4.6 every 10 years after the age of 45. The different types of dementia present at different ages, with frontotemporal dementia encompassing 10.2% of dementias in patients less than 65, whereas only accounting for 2.7% of dementias in patients over 65.Reference Hogan, Jetté and Fiest 1 - Reference Fiest, Roberts and Maxwell 3 Higher formal education levels have been identified as potentially protective against dementia, although the mechanism has only been theorized to this point.Reference Ngandu, von Strauss and Helkala 15 Sleep concerns are common in older adults, regardless of cognitive status.Reference Grace, Walker and McKeith 16 Some of the psychological causes of sleep disturbance, like depression and worry are frequently implicated in the “worried well” patients. However, sleep concerns have also been noted in dementia patients.Reference Menon and Larner 6 , Reference Foley, Ancoli-Israel, Britz and Walsh 17 Depression has also been implicated as a risk factor for memory concerns. Patients with MCI who also have depression are twice as likely to develop Alzheimer’s disease.Reference Modrego and Ferrández 18 The Center for Epidemiologic Studies Depression Scale (CES-D) is a screening tool used to evaluate depression.Reference Andresen, Malmgren, Carter and Patrick 19
The objective of the present study is to identify features of “worried well” patients to better identify those more likely to be cognitively normal. There has been an increasing number of referrals to specialists for memory concerns.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 However, the diagnosed dementia rates are not increasing proportional to the referral rates, leading to what has been described as a “diagnosis gap”.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 The rate of referral for memory concerns is growing more quickly than the rate of dementia, further highlighting the need for better indicators of dementia risk.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 It has been shown that a large proportion of patients with SMC will not convert to having objective memory concerns.Reference Mendonça, Alves and Bugalho 20 This paper concluded that further investigation needs to be done to better define risk features of these patients so that they are not over-investigated and triaged appropriately.Reference Mendonça, Alves and Bugalho 20 By identifying those at lower risk of having dementia, specialist resources can be better used.Reference Menon and Larner 6 , Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 , Reference Morgan, Crossley and Kirk 21 Limited specialist access is a challenge in rural areas.Reference Morgan, Crossley and Kirk 21
Methods
In total, 375 consecutive patients seen at a rural and remote memory clinic (RRMC) between March 2004 and October 2015 were included in this analysis. The University of Saskatchewan’s Rural and Remote Memory Clinic provides a one-stop interdisciplinary assessment for patients with memory concerns from across Saskatchewan. At the initial visit, patients are seen by a neurologist, a physiotherapist, a dietitian, a nurse and undergo neuropsychological testing.Reference Morgan, Crossley and Kirk 21 Each patient receives a standard work-up for reversible/vascular causes of memory concerns which includes: a complete blood count (CBC), electrolytes including calcium, thyroid stimulating hormone (TSH), vitamin B12, a non-contrast CT scan of their head and other investigations when indicated. More detailed information about the clinic can be found in previous publications.Reference Morgan, Crossley and Kirk 21 - Reference Verity, Kirk, Morgan and Karunanayake 31 Data collected at the initial visit include: age, sex, years of formal education, MMSE score from the initial RRMC visit, CES-D depression scores, Self-Rating of Memory Scale, alcohol consumption, marital status, hours per week of work, past medical history, sleep concerns, possession of a driver’s licence, and information on a family history of memory concerns. By the end of the day, patients are given a diagnosis as agreed upon by the assessment team. A patient received a diagnosis of “worried well” if all the following criteria were met: they had no clinical evidence of a neurologic disease, they had normal neuro-imaging, and if they had normal age, sex and education adjusted performance on neuropsychological testing. The neuropsychological battery includes measures of attention, speeded mental processing, language, visuospatial abilities, executive function, memory for stories, a word list, and a complex figure. The repeatable battery for the assessment of neuropsychological status (RBANS) is a brief assessment tool designed to identify mild to severe forms of dementia in older adults.Reference Strauss, Sherman and Spreen 32 We then categorized patients into one of two groups based on their neurologic diagnosis, “worried well” (cognitively normal, N=81) or “other” (includes all neurologic diagnosis, N=294). The “other” group included patients given a diagnosis of MCI. A diagnosis of MCI required: memory complaints corroborated by a collateral history, objective signs of memory impairment, relatively preserved functional abilities and that the patient’s presentation does not meet criteria for dementia.Reference Petersen 33 To directly compare cognitively normal patients and dementia patients we did further analysis. This second analysis used the same set of “worried well” patients (N=81) and the subgroup of patients diagnosed with Alzheimer’s disease as per NINCDS-ADRDA criteria (N=146) from the “other” group.Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan 34 The same patient information was isolated, and then re-analyzed comparing these two groups. Statistical analyses were conducted using SPSS version 24. 35 Descriptive analyses were completed using frequencies, measures of central tendency, variability and χ2 test of associations. The two groups (worried well vs. other/worried well vs. Alzheimer’s disease group) were compared using independent sample t-tests for continuous variables and χ2 tests for categorical variables. When the cell sizes were small, χ2 exact p values were reported. Effect size was then calculated using Cohen’s d. Ethics approval was obtained from the University of Saskatchewan Biomedical Research Ethics Board.
Results
In total, 375 patients who underwent an initial clinical assessment were included in this analysis. When comparing the “worried well” group (N=81) and the “other” group (N=294), the “worried well” group was significantly younger (Table 1). Other significant differences included more formal education, more self-reported alcohol consumption, and higher MMSE scores in the “worried well” group (Table 1). Self-reported memory concerns showed no difference between the “worried well” group and the “other” group. There was no statistically significant difference in self-reported family history of memory concerns or dementia between these two groups. There was a significant difference between self-reported previous history of psychiatric or psychologic problems, with the “worried well” group more frequently having a previous diagnosis or problem. The “worried well” group also had a significantly higher CES-D depression screening score, with a moderate effect size (Cohen’s d=0.57). The full comparison between the “worried well” and “other” groups is presented in Table 1.
Table 1 Characteristics of normal and all other patientsFootnote *
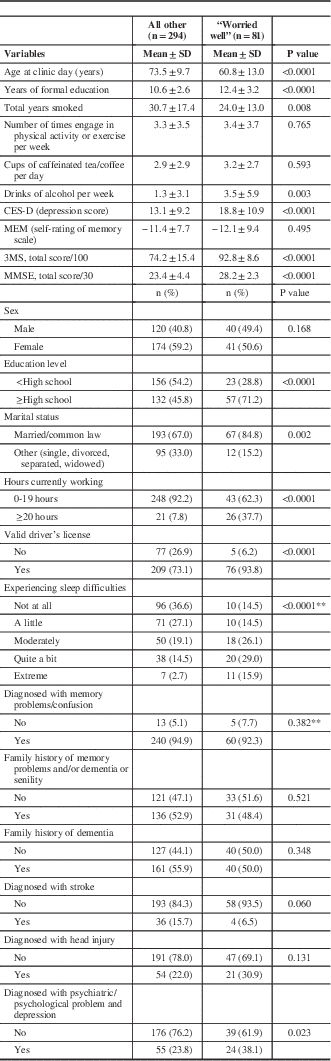
CES-D=Center for Epidemiologic Studies Depression Scale; MEM=memory; MMSE=Mini-Mental Status Examination; 3MS=Modified Mini-Mental State.
* All variables have missing values except age.
** Due to small expected values exact test p value was reported.
Of the 375 total patients, 227 patients were included in the second analysis which compared the same “worried well” group (N=81) to the patients who received a diagnosis of Alzheimer’s disease (N=146). This comparison had similar differences as in the first analysis. The full comparison between the “worried well” and “Alzheimer’s Disease” groups is detailed in Table 2.
Table 2 Characteristics of Alzheimer’s disease (AD) and normal patientsFootnote *
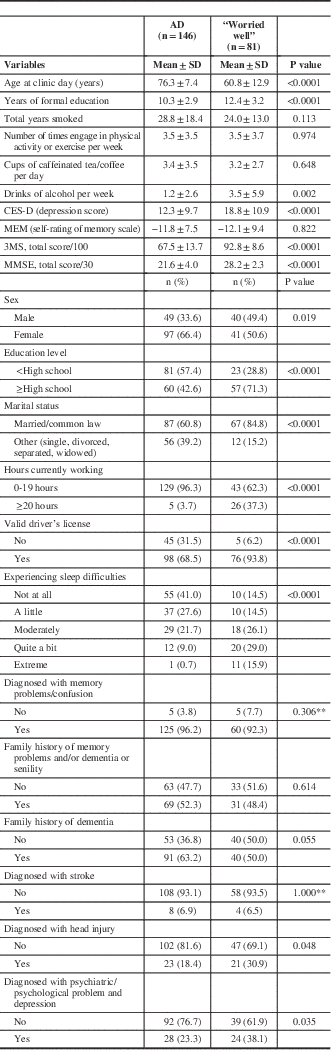
CES-D=Center for Epidemiologic Studies Depression Scale; MMSE=Mini-Mental Status Examination.
* All variables have missing values except age.
** Due to small expected values exact test p value was reported.
This data are mostly self-reported, as it is acquired through a questionnaire completed by patient and family at the patient’s initial clinic assessment. As a result, all variables other than age have missing values. The number of values for each variable is included in the previously mentioned Tables 1 and 2. Breakdown of the “other” diagnoses can be found in Table 3.
Table 3 “Other” diagnosis

Other group includes: corticobasal degeneration, hydrocephalus, Parkinson’s dementia, medication side effects, Huntington’s disease, Fragile X associated dementia, Herpes encephalitis, hypoxic ischemic encephalopathy, multiple systems atrophy, progressive supranuclear palsy.
The initial neuropsychological data are outlined in Tables 4 and 5. Comparison of neuropsychological test results between the “worried well” and the “other” group is presented in Table 4. Comparison between the “worried well” and Alzheimer’s Disease patients is outlined in Table 5.
Table 4 Comparison of clinic day neuropsychological data: “worried well” versus Alzheimer’s disease (AD)
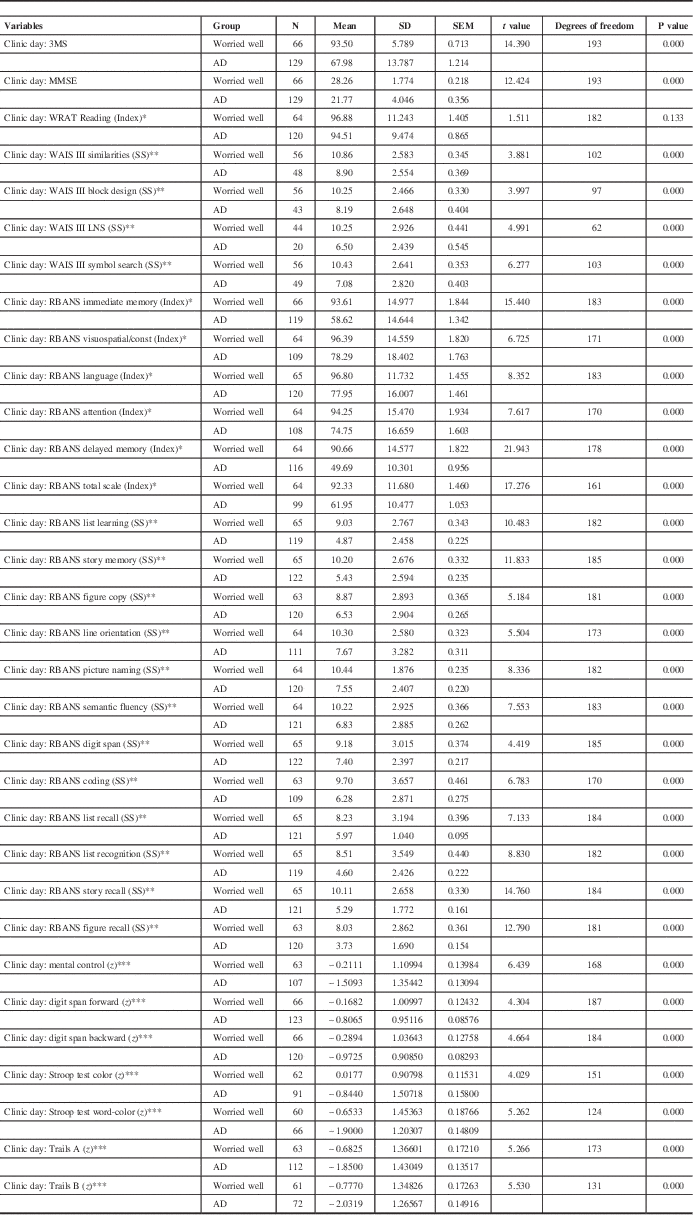
The repeatable battery for the assessment of neuropsychological status (RBANS) is a brief assessment tool designed to identify mild to severe forms of dementia in older adults.Reference Strauss, Sherman and Spreen 32 LNS=Letter Number Sequencing; WAIS=Wechsler Adult Intelligence Scale; WRAT=Wide Range Achievement Test.
* Index score is a composite score with a mean (M) of 100 and a SD of 15.
** Scaled score (SS) is a linear transformation of raw scores with M=10, SD=3.
*** z Score is a linear transformation of raw scores with M=0, SD=1.
Table 5 Comparison of clinic day neuropsychological data: “worried well” versus “other”
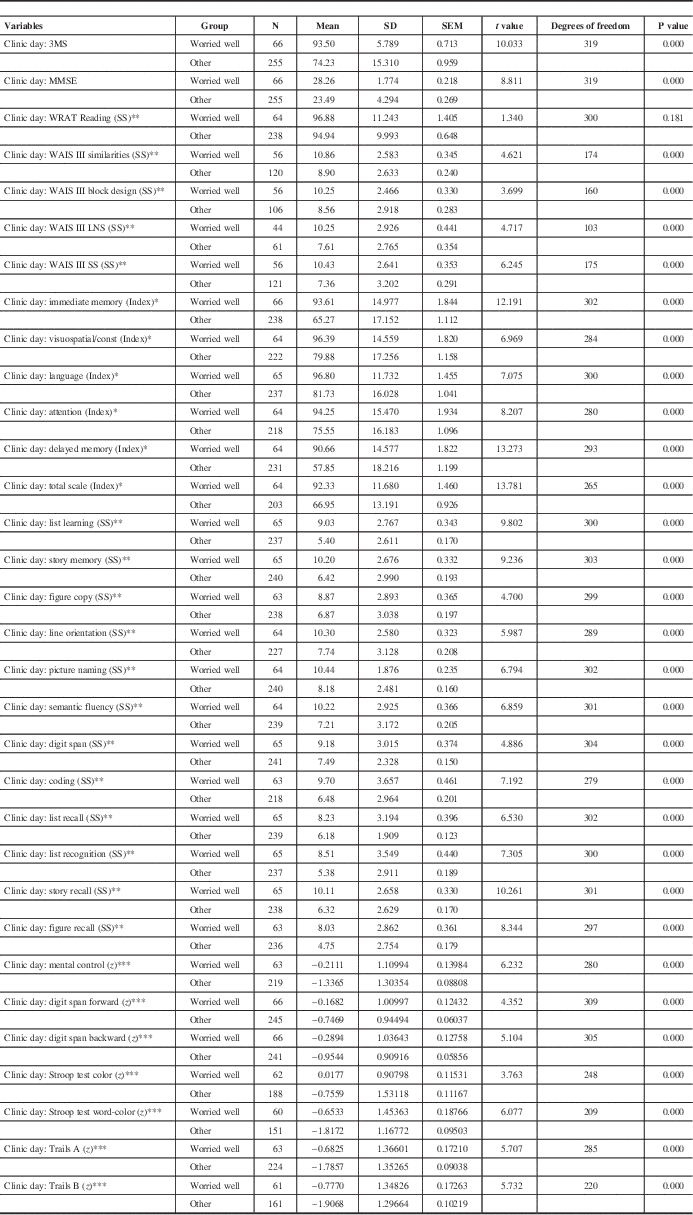
The repeatable battery for the assessment of neuropsychological status (RBANS) is a brief assessment tool designed to identify mild to severe forms of dementia in older adults.Reference Strauss, Sherman and Spreen 32
* Index score is a composite score with a mean (M) of 100 and a SD of 15.
** Scaled score is a linear transformation of raw scores with M=10, SD=3.
*** z Score is a linear transformation of raw scores with M=0, SD=1.
Discussion
With the increasing awareness surrounding degenerative neurologic disease, patients with self-expressed memory concerns are often cognitively normal.Reference Cutler 4 This has previously been described as a “worried well” patient, often someone who has a family member or friend with a memory concern who then is more aware of common memory lapses that every individual occasionally makes.Reference Ahmed, Mitchell, Arnold, Dawson, Nestor and Hodges 7 With no conclusive diagnostic test for AD, evaluating memory concerns is a somewhat subjective task. In order to better address the issues of the “worried well” we sought to identify trends in their characteristics so as to better identify who is at greater risk of a degenerative disease and who might be more likely to benefit from reassurance and education on cognitive aging.
With over 20% of patients at the RRMC being diagnosed as cognitively normal, we have a fair sample size to assess differences. Of the many significant differences between the cognitively normal and other groups, age and MMSE stand out as highly valuable clinical indicators. Alzheimer’s disease classically presents later in life, and statistically most of the “worried well” patients were in their early sixties, whereas those with dementia had a mean age of 76.3 years. There was a significant difference in age between the “normal” or “worried well” group and both the “other” group and the “Alzheimer’s Disease” group. Although other forms of dementia do indeed occur earlier, these diseases are not as common as Alzheimer’s Disease.
We found that the cognitively normal patients tended to have more years of formal education. It may be that those with higher levels of education are more aware of the impact of dementia, and as a result are more likely to be “worried well” or that they are more impacted by normal cognitive changes with aging.Reference Ngandu, von Strauss and Helkala 15 The cognitively normal patients were also more likely to be working part-time or more. The demands of work may make small lapses in memory more apparent resulting in more awareness of one’s own mistakes leading to this category of the “worried well” patient. Alcohol intake also differed between the two groups, with the “worried well” patients having higher rates of consumption. However, both groups were still within the recommended consumption guidelines. We wondered about the potential cognitive impacts alcohol consumption had on these patients, although causation or association with memory concerns is not clear. The “worried well” patients were more likely to have a driver’s licence, and this fits with their lower age and increased prevalence of part-time or more work.
Psychiatric illness or psychological problems play an important role in evaluating patients with memory concerns.Reference Modrego and Ferrández 18 , Reference Kosteniuk, Morgan and O’Connell 36 , Reference Gatz, Tyas, John and Montgomery 37 Psychiatric problems can impair memory, and may be implicated as the etiology behind the memory problems.Reference Modrego and Ferrández 18 , Reference Kosteniuk, Morgan and O’Connell 36 , Reference Gatz, Tyas, John and Montgomery 37 However, some psychiatric problems co-occur with memory concerns, as depression is common in Alzheimer’s disease.Reference Foley, Ancoli-Israel, Britz and Walsh 17 , Reference Petersen 33 , Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan 34 Therefore, it is interesting to note the significant difference between CES-D depression screening scores in the “worried well” and both the “other” and “Alzheimer’s disease” groups. A recent study was done at this RRMC to look at depression prevalence between no cognitive impairment (NCI) patients and dementia patients.Reference Kosteniuk, Morgan and O’Connell 36 This study found that depression was more common in the NCI patients.Reference Gatz, Tyas, John and Montgomery 37 However, a study done in 2005 looking at CES-D scores in Alzheimer’s disease patients found that higher scores were found in dementia patients.Reference Gatz, Tyas, John and Montgomery 37 It was concluded that a CES-D score of 21 was a significant statistical predictor of dementia.Reference Gatz, Tyas, John and Montgomery 37 Another study found that SMC were more strongly associated with depression than with cognitive impairment.Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan 34 Our data shows a mean CES-D score of 12.3 in dementia patients, and a significantly higher mean score of 18.8 in the “worried well” (Cohen’s d=0.63). This creates an interesting contrast, as depression may be an early symptom of dementia but can also independently contribute to memory concerns.Reference Kosteniuk, Morgan and O’Connell 36 , Reference Gatz, Tyas, John and Montgomery 37 Depression and dementia may also share common behavioral symptoms such as lack of interest in activities or apathy.Reference Kosteniuk, Morgan and O’Connell 36 , Reference Gatz, Tyas, John and Montgomery 37 Our data appears to reflect higher CES-D scores in “worried well” patients, and may be attributed to part of their subjective memory complaint. This data supports the supposition that depression may be more likely to cause independent memory concerns, rather than be an early sign of dementia, depending on the patient’s other risk factors.Reference Montejo Carrasco, Montenegro-Peña and López-Higes 38 Anxiety or worry about getting dementia, coupled with increased awareness surrounding the disease likely leads many patients to become more aware of lapses in memory that are normal rather than a sign of a degenerative disease. The “Alzheimer’s disease” group had significantly less concerns with sleep than the “worried well” group. This may be associated with psychologic factors discussed previously that seem to be prevalent in “worried well” patients or poor sleep may have contributed to memory symptoms. The “worried well” group also had greater incidence of previous psychiatric diagnosis. This is an interesting point, as psychiatric illness may be a predictor of SCI. Psychiatric illness may independently cause memory concerns, or psychiatric symptoms might overlap with behaviors that are an early sign of dementia, such as apathy. Therefore, further investigation is warranted. Taken with the fact that the “worried well” patients were generally younger; it may be that depressive symptomatology is a major cause of memory concerns in younger patients. Therefore, it is essential to evaluate mood and mental health when discussing memory concerns with patients.
A number of variables we examined showed no significant difference between groups leading us to question their validity in characterizing a patient as “worried well” or not. Most notable was the lack of significant difference in family history of memory concerns or dementia between the two groups. This could be because dementia is so common. Stroke and head injuries were also not more prevalent in one group or the other.
Study limitations include a lack of biomarkers that have been studied as predictors of dementia, and a cross-sectional study design where follow-up is not reported. Longitudinal follow-up would be an interesting topic of future research papers.
Conclusions
Overall, we observed a pattern of differences unfold between the “worried well” patients and those with cognitive disease. No one variable is pathognomonic of a “worried well” patient. However, taking age, MMSE score, psychiatric/psychological condition, substance use, sleep concerns and clinical picture into consideration when evaluating a patient may help with clinical decision making. The over-arching theme of increased awareness, exposure to the disease and psychological factors help illustrate a typical “worried well” patient. By better identifying the “worried well” we can make better use of resources, like specialist referrals, and improve patient care by providing appropriate management aimed at the underlying cause of the concern.
Acknowledgment
The authors would like to acknowledge the Rural and Remote Memory Clinic at the University of Saskatchewan for the support with this project.
Statement of Authorship
All mentioned authors contributed in the design, analysis, and execution of this project.
Disclosure
Ryan Verity, Andrew Kirk, Megan E. O’Connell, Chandima Karunanayake, and Debra G. Morgan do not have anything to disclose.









