The guideline was developed by a panel of 25 experts from across Canada, with representatives from infectious diseases, primary care, emergency medicine, public health, pharmacy, nursing, and the community. Funding for the work was provided by the Canadian Institutes of Health Research (CIHR; grant number PCS 142089), with in-kind support from the CIHR Canadian HIV Trials Network and a New Investigator Award from the CIHR/Ontario HIV Treatment Network (D.H.S.T.).
Non-occupational post-exposure prophylaxis
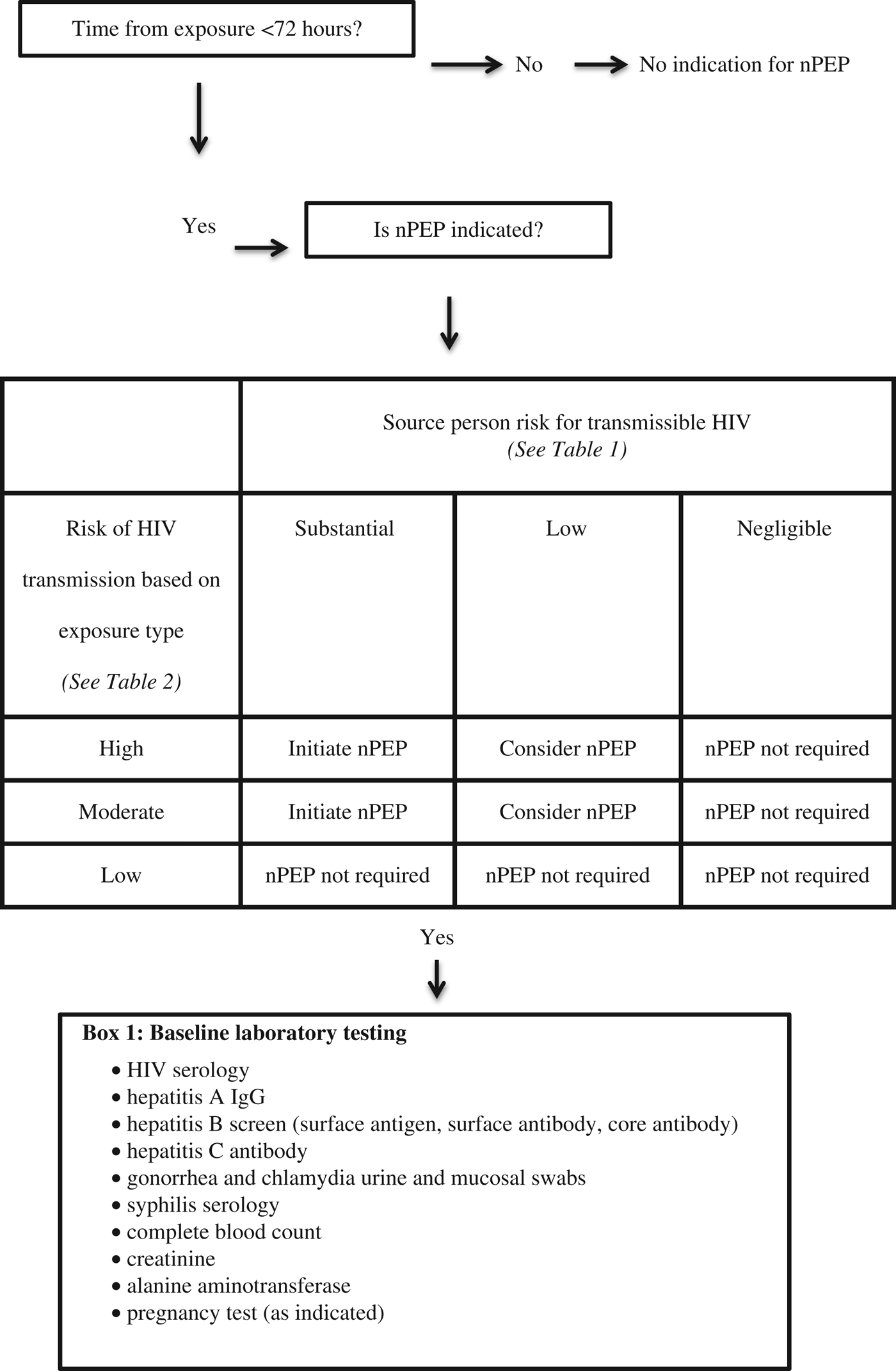
The guideline recommends that nPEP be started in patients who are HIV negative and present within 72 hours of an exposure that is of a moderate or high risk and involves a source person who is HIV positive or at risk for having transmissible HIV (see Table 1). HIV nPEP is not recommended in any other scenarios nor is it recommended beyond 72 hours from the exposure.Reference Tan, Hull and Yoong6
The risk for a given exposure type is based on estimates of per-act HIV transmission risk from a known HIV positive source. Receptive anal sex carries the highest risk for transmission, followed (in decreasing order of risk) by needle sharing, insertive anal sex, receptive vaginal sex, and insertive vaginal sex. nPEP is not indicated after oral sex. (See Table 2).
Determining the involved source person’s risk for having transmissible HIV in the ED is often difficult. Very rarely is the source person available for interviewing or HIV testing, and, often, the source is not known to the patient. In these cases, a determination of whether the source is at high epidemiologic risk for HIV must be made. In Canada, HIV prevalence is elevated among MSM, PWID, individuals from HIV-endemic countries, and certain indigenous populations.Reference Bourgeois, Edmunds and Awan1 The HIV epidemic varies geographically across Canada, however, and clinicians should be aware of local epidemiology. In addition, caution is advised when applying epidemiologic constructs to individuals, as this may contribute to stigma and discrimination and may not apply to the source person in question.
The guidelineReference Tan, Hull and Yoong6 provides recommendations for nPEP after consensual exposures only and does not include specific directives for treating patients after a sexual assault. In centres where sexual assault services are available, patients should be referred accordingly.Reference Tan, Hull and Yoong6 If these services are not available, the ED physician should consider that circumstances often associated with assault (trauma or bleeding, multiple assailants, or possible presence of a sexually transmitted infection [STI] in the assailant) increase the risk for HIV transmission.
Another scenario not addressed in the guidelineReference Tan, Hull and Yoong6 is the patient who presents after a needlestick injury from a discarded or abandoned needle (found in a park or garbage). There have not been any documented cases of HIV infection from such injuriesReference Dominguez, Smith and Vasavi12 and, in general, they are a very low risk for transmission of HIV because the needles in question are often small-bore needles, there is usually minimal blood in the syringe, and HIV does not survive outside the body for prolonged periods.
Certainly not all patient presentations fit neatly into the scenarios defined by the guideline,Reference Tan, Hull and Yoong6 and nPEP should involve shared decision making with the patient. Each case should be considered on an individualized basis, and if there is uncertainty about whether nPEP is indicated, the emergency physician should obtain subspecialty support.
In addition to HIV serology, baseline laboratory investigations in the ED should include thorough STI testing (urine and mucosal swabs for gonorrhea and chlamydia; serology for syphilis and hepatitis A, B, and C), complete blood count, creatinine, alanine aminotransferase, and pregnancy testing as applicable. Emergency physicians should make onward referrals to other providers who can conduct follow-up testing 12 weeks after the exposure and manage other considerations regarding special populations, ongoing monitoring while on nPEP, and indications for stopping nPEP.Reference Tan, Hull and Yoong6
When indicated, medications should be started as soon as possible. When patients are dispensed the full 28-day course of nPEP rather than a starter pack of medications, PEP completion rates are better and there are fewer PEP refusals.Reference Ford, Venter, Irvine, Beanland and Shubber13 However, if the need for continued prophylaxis will be reassessed pending source testing, if there is a concern for drug resistance, or if drug coverage does not include nPEP, starter packs dispensed in the ED are recommended.Reference Tan, Hull and Yoong6
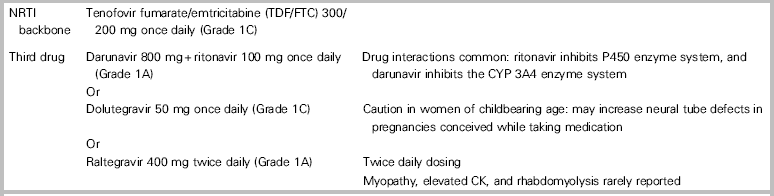
Twenty-eight-day nPEP regimens include two nucleoside reverse transcriptase inhibitors (tenofovir disoproxil fumarate [TDF]/emtricitabine [FTC] 300/200 mg orally once daily), plus a third drug (darunavir 800 mg orally once daily plus ritonavir 100 mg orally once daily, Grade 1A) dolutegravir 50 mg orally once daily, Grade 1C; or raltegravir 400 mg orally twice daily, Grade 1A).Reference Tan, Hull and Yoong6 See Table 3.
Table 3 Preferred nPEP drug regimens*
Pre-exposure prophylaxis
PrEP is the use of TDF/FTC 300/200 mg orally either once daily or “on demand” on the days surrounding sexual encounters to prevent transmission of HIV. The guidelineReference Tan, Hull and Yoong6 lists indications for the use of PrEP by MSM and transgender women at high risk for infection, as well as at-risk HIV-negative partners in heterosexual serodiscordant relationships, and certain PWID.
Because individuals taking PrEP should be assessed clinically at regular intervals, the ED is not the appropriate setting for initiation of PrEP. It is important, however, that emergency physicians be able to recognize patients who may be candidates for PrEP (for instance, gay, bisexual, or other MSM with recurrent use of nPEP, rectal bacterial STIs, or early syphilis) and refer these patients to their primary care physician or another suitably trained provider for consideration of PrEP initiation.







BACKGROUND
In the last decade, the number of new human immunodeficiency virus (HIV) infections in Canada decreased yearly from 2,599 in 2008 to 2,053 in 2014. Since 2014, however, there has been an uptick in the annual incidence, and in 2016, there were 2,344 new cases of HIV reported in Canada.Reference Bourgeois, Edmunds and Awan1 While it is theoretically possible to acquire HIV after occupational exposure (e.g., a needlestick injury in a health care setting), new HIV infections occur almost exclusively as a result of non-occupational exposures (e.g., sexual contact or needle sharing). Only one case of HIV transmission from an occupational exposure has been confirmed in the United States since 1999.Reference Joyce, Kuhar and Brooks2
New HIV infections are disproportionately concentrated among certain populations. Recent Canadian data revealed that nearly one-half of all new infections (44.1%) occur among men who have sex with men (MSM). Heterosexual contact represents the next most common route of transmission (32.3%), with one-third of these cases (10.5%) occurring in people from HIV-endemic countries. Finally, 15.1% of new HIV infections are identified in people who inject drugs (PWID), more than one-half of whom are indigenous. The remaining new infections result from a combination of injection drug use and sexual contact between MSM and from unspecified exposure routes.Reference Bourgeois, Edmunds and Awan1
While emergency departments (ED) serve as an important resource for timely access to HIV post-exposure prophylaxis (PEP), the literature suggests that emergency physicians have not felt confident determining the need for PEP when patients present after sexual contact or the use of injection drugs.Reference McCausland, Linden and Degutis3 Consistent with this finding, recent data show that emergency physicians under prescribe HIV PEP when indicated: in a review of patients presenting to a Vancouver ED, more than one-quarter of those who should have received HIV PEP after a high-risk non-occupational exposure (based on 2005 Centers for Disease Control and Prevention recommendationsReference Smith, Grohskopf and Black4) did not.Reference O’Donnell, Bhate and Grafstein5
In 2017, a Canadian guideline on HIV pre-exposure prophylaxis (PrEP) and non-occupational post-exposure prophylaxis (nPEP)Reference Tan, Hull and Yoong6 was published to provide clinicians with an evidence-based approach for assessing the risk for HIV, providing antiretroviral medications as a preventative measure, conducting baseline and follow-up testing, and monitoring medication safety. The guidelineReference Tan, Hull and Yoong6 is the first of its kind in Canada and is broadly consistent with guidelines from Europe, the United Kingdom, the United States, and Australia.Reference Brady, Rodger and Asboe7–10 In this article, we outline the key points that are relevant to the practice of emergency medicine in Canada, with a focus on determining which patients should be treated with nPEP.
DESCRIPTION
The guideline was developed by a panel of 25 experts from across Canada, with representatives from infectious diseases, primary care, emergency medicine, public health, pharmacy, nursing, and the community. Funding for the work was provided by the Canadian Institutes of Health Research (CIHR; grant number PCS 142089), with in-kind support from the CIHR Canadian HIV Trials Network and a New Investigator Award from the CIHR/Ontario HIV Treatment Network (D.H.S.T.).
Methods for development of the guideline are described in detail.Reference Tan, Hull and Yoong6 The Grading of Recommendation, Assessment, Development and Evaluation (GRADE) systemReference Atkins, Best and Briss11 was used to specify two categories of strength of recommendation and four categories of quality of evidence for each of the recommendations (Appendix 1).
Non-occupational post-exposure prophylaxis
The guideline recommends that nPEP be started in patients who are HIV negative and present within 72 hours of an exposure that is of a moderate or high risk and involves a source person who is HIV positive or at risk for having transmissible HIV (see Table 1). HIV nPEP is not recommended in any other scenarios nor is it recommended beyond 72 hours from the exposure.Reference Tan, Hull and Yoong6
Table 1 Risk that a person has transmissible HIV infectionReference Gray, Wawer and Brookmeyer15–Reference Cohen, Chen and McCauley17
HIV=human immunodeficiency virus; STI=sexually transmitted infection; VL=viral load, copies/mL.
The risk for a given exposure type is based on estimates of per-act HIV transmission risk from a known HIV positive source. Receptive anal sex carries the highest risk for transmission, followed (in decreasing order of risk) by needle sharing, insertive anal sex, receptive vaginal sex, and insertive vaginal sex. nPEP is not indicated after oral sex. (See Table 2).
Table 2 Risk of HIV transmission per act by exposure type from an HIV-positive sourceReference Patel, Borkowf and Brooks14
Determining the involved source person’s risk for having transmissible HIV in the ED is often difficult. Very rarely is the source person available for interviewing or HIV testing, and, often, the source is not known to the patient. In these cases, a determination of whether the source is at high epidemiologic risk for HIV must be made. In Canada, HIV prevalence is elevated among MSM, PWID, individuals from HIV-endemic countries, and certain indigenous populations.Reference Bourgeois, Edmunds and Awan1 The HIV epidemic varies geographically across Canada, however, and clinicians should be aware of local epidemiology. In addition, caution is advised when applying epidemiologic constructs to individuals, as this may contribute to stigma and discrimination and may not apply to the source person in question.
The guidelineReference Tan, Hull and Yoong6 provides recommendations for nPEP after consensual exposures only and does not include specific directives for treating patients after a sexual assault. In centres where sexual assault services are available, patients should be referred accordingly.Reference Tan, Hull and Yoong6 If these services are not available, the ED physician should consider that circumstances often associated with assault (trauma or bleeding, multiple assailants, or possible presence of a sexually transmitted infection [STI] in the assailant) increase the risk for HIV transmission.
Another scenario not addressed in the guidelineReference Tan, Hull and Yoong6 is the patient who presents after a needlestick injury from a discarded or abandoned needle (found in a park or garbage). There have not been any documented cases of HIV infection from such injuriesReference Dominguez, Smith and Vasavi12 and, in general, they are a very low risk for transmission of HIV because the needles in question are often small-bore needles, there is usually minimal blood in the syringe, and HIV does not survive outside the body for prolonged periods.
Certainly not all patient presentations fit neatly into the scenarios defined by the guideline,Reference Tan, Hull and Yoong6 and nPEP should involve shared decision making with the patient. Each case should be considered on an individualized basis, and if there is uncertainty about whether nPEP is indicated, the emergency physician should obtain subspecialty support.
In addition to HIV serology, baseline laboratory investigations in the ED should include thorough STI testing (urine and mucosal swabs for gonorrhea and chlamydia; serology for syphilis and hepatitis A, B, and C), complete blood count, creatinine, alanine aminotransferase, and pregnancy testing as applicable. Emergency physicians should make onward referrals to other providers who can conduct follow-up testing 12 weeks after the exposure and manage other considerations regarding special populations, ongoing monitoring while on nPEP, and indications for stopping nPEP.Reference Tan, Hull and Yoong6
When indicated, medications should be started as soon as possible. When patients are dispensed the full 28-day course of nPEP rather than a starter pack of medications, PEP completion rates are better and there are fewer PEP refusals.Reference Ford, Venter, Irvine, Beanland and Shubber13 However, if the need for continued prophylaxis will be reassessed pending source testing, if there is a concern for drug resistance, or if drug coverage does not include nPEP, starter packs dispensed in the ED are recommended.Reference Tan, Hull and Yoong6
Twenty-eight-day nPEP regimens include two nucleoside reverse transcriptase inhibitors (tenofovir disoproxil fumarate [TDF]/emtricitabine [FTC] 300/200 mg orally once daily), plus a third drug (darunavir 800 mg orally once daily plus ritonavir 100 mg orally once daily, Grade 1A) dolutegravir 50 mg orally once daily, Grade 1C; or raltegravir 400 mg orally twice daily, Grade 1A).Reference Tan, Hull and Yoong6 See Table 3.
Table 3 Preferred nPEP drug regimens*
CK=creatine kinase; NRTI=nucleoside reverse transcriptase inhibitors; nPEP=non-occupational post-exposure prophylaxis.
* A thorough medication history (including prescription drugs, supplements, and herbal preparations) should be taken prior to selecting an nPEP regimen because of the potential for drug-drug interactions.
Pre-exposure prophylaxis
PrEP is the use of TDF/FTC 300/200 mg orally either once daily or “on demand” on the days surrounding sexual encounters to prevent transmission of HIV. The guidelineReference Tan, Hull and Yoong6 lists indications for the use of PrEP by MSM and transgender women at high risk for infection, as well as at-risk HIV-negative partners in heterosexual serodiscordant relationships, and certain PWID.
Because individuals taking PrEP should be assessed clinically at regular intervals, the ED is not the appropriate setting for initiation of PrEP. It is important, however, that emergency physicians be able to recognize patients who may be candidates for PrEP (for instance, gay, bisexual, or other MSM with recurrent use of nPEP, rectal bacterial STIs, or early syphilis) and refer these patients to their primary care physician or another suitably trained provider for consideration of PrEP initiation.
SUMMARY
Patients with non-occupational exposures to HIV often present to the ED and are cared for by emergency physicians. Preventing the transmission of HIV is of social and economic importance, given the high cost of treating HIV and the young age at which most infections occur (age 30–39 yearsReference Bourgeois, Edmunds and Awan1). Canadian research has shown that there are patients who have had high-risk exposures and were discharged without appropriate treatment.Reference O’Donnell, Bhate and Grafstein5 The goal of this article was to update emergency physicians on the new Canadian guideline for nPEP and PrEP to enable the highest standard of care possible. (Figure 1)
Figure 1. Algorithm for HIV nPEP assessment
Competing interests
None declared.
SUPPLEMENTARY MATERIALS
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2018.462