Introduction
Escherichia coli is as one of the most important enteric human pathogens worldwide [Reference Kotloff1]. Strains of E. coli causing enteric diseases are collectively designated diarrhoeagenic (DEC) and are currently divided into six main categories or pathotypes based on defined virulence attributes. The known DEC pathotypes are named enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), Shiga toxin-producing E. coli (STEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC) and diffusely adherent E. coli (DAEC) [Reference Croxen2].
EPEC and EAEC induce diarrhoea through their ability to adhere to host intestinal mucosa, leading to the formation of attaching and effacing (A/E) lesions in the case of EPEC and the aggregative adhesion (AA) pattern in the case of EAEC [Reference Croxen2, Reference Estrada-Garcia and Navarro-Garcia3]. Genes such as eae for A/E lesion and aaf (AA fimbriae) for AA, among others, are responsible for the production of these featured adhesion phenotypes [Reference Estrada-Garcia and Navarro-Garcia3, Reference Hernandes4]. As pathogenic groups both EPEC and EAEC are subdivided in typical and atypical strains. For EPEC, this division is based upon the presence of EAF plasmid (pEAF) in typical (tEPEC) strains and its absence in atypical (aEPEC) ones [Reference Hernandes4]. The pEAF contains in its structure an operon termed bfp, which is responsible for the production of a type IV pilus named bundle-forming pilus (BFP). BFP is thought to be involved in bacteria to bacteria interactions during the host colonization by EPEC [Reference Hernandes4]. The occurrence of gene aggR defines typical EAEC strains while atypical EAEC are devoid of this marker [Reference Croxen2]. Gene aggR is regarded as a major transcriptional regulator of many of the genes responsible for EAEC virulence factors production [Reference Estrada-Garcia and Navarro-Garcia3]. STEC and ETEC damage the host mainly by elaborating and secreting toxins [Reference Croxen2]. STEC produces Shiga toxins (Stx). There are two distinct Stx types, Stx1 and Stx2 [Reference Hernandes4] with 10 subtypes, 1a, 1c and 1d for Stx1, and 2a, 2b, 2c, 2d, 2e, 2f and 2g for Stx2 [Reference Parsot5]. ETEC produces thermolabile (LT) and thermostable (ST) enterotoxins. Both LT and ST toxins can also be divided into the distinct antigenic types LT-I and LT-II and ST-I and STII. Furthermore, ST-I may present human (STh) and porcine (STp) variant forms [Reference Croxen2]. EIEC phenotypically resemble the genus Shigella. They are capable of invading the host intestinal mucosa and this invasive behaviour relies on a complex array of effector molecules which are employed by the bacteria in order to penetrate, evade immune response and replicate within intestinal cells [Reference Parsot5]. The consequent inflammatory response triggered against the invasion process leads to the damage of the intestinal epithelia, characteristic of the bacillary dysentery [Reference Croxen2].
In addition to E. coli, another species within the genus Escherichia, E. albertii, can also be a human pathogen. E. albertii was isolated for the first time from a diarrhoeic child in Bangladeshi and misidentified as Hafnia alvei [Reference Hyma6]. Currently, E. albertii is considered an ‘emerging’ human enteric pathogen. Similarly to EPEC, E. albertii also harbours the eae gene and thus may produce A/E lesions. Some isolates may possess additional virulence determinants like cytolethal distending and Stx toxins [Reference Ooka7].
EPEC, EAEC and ETEC are leading bacterial causes of acute childhood diarrhoea worldwide [Reference Platts-Mills8]. EPEC and EAEC have also been implicated in prolonged diarrhoeal diseases and ETEC along with EAEC are agents of the so-called ‘traveller diarrhoea’. On the other hand, STEC strains have been linked with large outbreaks of diarrhoea, and with the occurrence of haemorrhagic colitis and haemolytic uremic syndrome (HUS) [Reference Paton and Paton9].
In Brazil, the presence of DEC strains has been investigated in young children, in studies conducted at specific geographic locations [Reference Moreno10]. However, there are no reports assessing the occurrence of DEC pathotypes from official surveillance programmes and involving patients from all age groups. Given the heterogeneous nature of DEC strains and their ability to emerge in new pathogenic forms through the gain or loss of genetic material [Reference Bielaszewska11], monitoring their virulence traits is of great utility as it can inform on outbreak detection. In order to provide useful epidemiologic data on the occurrence of DEC in Brazil, the present study aimed to describe the pathotypes and serotypes of E. coli and E. albertii strains associated with human infections.
Material and methods
Bacterial strains
The Brazilian Reference Laboratory for E. coli enteric infections Adolfo Lutz Institute (IAL) receives clinical isolates biochemically characterized as E. coli from several regional and local public health laboratories for molecular pathotype identification and serotyping. From January of 2011 to December of 2016, E. coli isolates representing 5047 cases of human infection, including two cases of HUS, were sent to our laboratory for this purpose. Of these, 82 cases had been previously analysed during the investigation of outbreaks of diarrhoea in the years of 2012 and 2013 [Reference Vieira12]. These cases were also included in this study as they contribute to the total cases recorded in the period of the present study. The cases encompassed subjects of all age groups. Due to the fact that commensal E. coli is the predominant facultative anaerobe in the human gut, for the identification of diarrhoeagenic strains, it is necessary to evaluate more than one E. coli-like colony from the same patient. In our laboratory, five to 10 E. coli colonies from each patient are routinely received for the characterization of DEC-specific virulence markers. If more than one colony of the same pathotype is found to be positive, only one colony is considered in each case. In the present study, cases of mixed infection (two distinct DEC pathotypes occurring in the same patient) were not considered. This study also employed reference strains serving as positive controls for each of the following DEC pathotypes: EPEC (E2369/48), EAEC (17-2), ETEC (H10407), STEC/EHEC (EDL933), EIEC (Shigella flexneri, CDC2a). The commensal E. coli K12:H5 served as a negative control for molecular and phenotypic procedures.
DEC pathotypes investigation
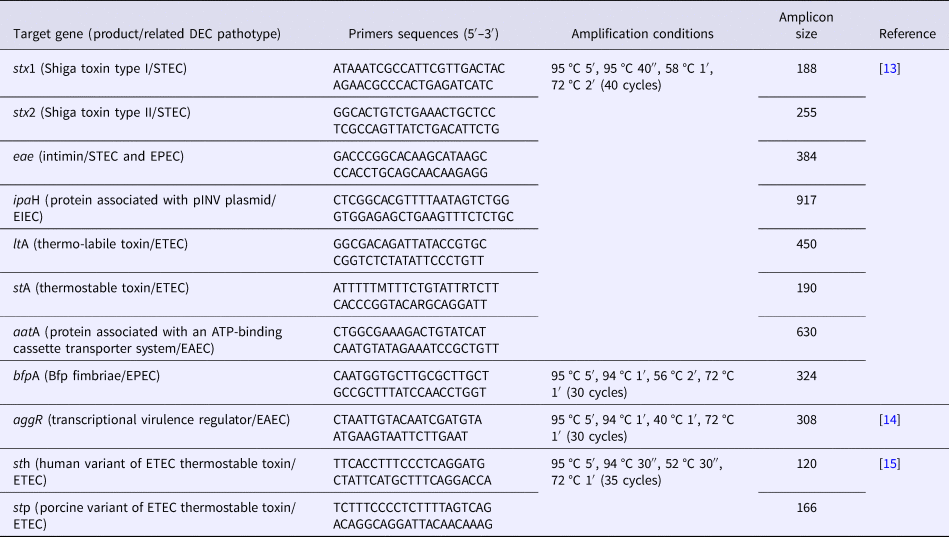
Screening for specific virulence genes (Table 1) defining the five most relevant DEC pathotypes (EPEC, EAEC, STEC, ETEC and EIEC) was performed by a multiplex PCR assay. For EPEC, the eae gene which is located in the pathogenicity island locus of enterocyte effacement (LEE) and is responsible for the production of the adhesin intimin was employed. For EAEC, the aatA gene encoding a protein related to an ATP-binding cassette transport system was used. For STEC, genes stx1 and stx2, which are bacteriophage-borne and related to the production of the Stx1 and Stx2 toxins respectively, were chosen. For ETEC, we used ltA and stA related to LT and ST toxins production, and for EIEC, the detection target was ipaH gene, which is associated with the invasion plasmid antigen H. Primers sequences and amplification parameters employed in the assays are described in Table 1. Template DNA for PCR reactions was produced by boiling bacterial suspensions from individual E. coli colonies cultivated on Tryptic Soy agar. After bacterial lysates preparation, five to 10 colonies from each patient were pooled and tested. If a given pool was positive, individual colonies forming this pool were retested with primers for the corresponding amplified gene. If a positive result was achieved, the corresponding colony was confirmed as positive.
Table 1. Primer sequences, target genes and amplification conditions employed in multiplex and individual PCR assays for characterizing DEC strains analysed in this study

E. albertii investigation
E. albertii was investigated by a triplex PCR assay recently described by Lindsey et al. This PCR targets cyclic di-GMP regulator gene (cdgR), DNA-binding transcriptional activator of cysteine biosynthesis gene (EAKF1_ch4033) and palmitoleoyl-acyl carrier protein-dependent acyltransferase gene (EFER_0790) allowing discrimination among E. coli, E. albertii and E. fergusonii [Reference Lindsey16].
Shiga toxin genes (stx) subtyping
Strains presenting stx1 and/or stx2 genes were subjected to stx subtyping by PCR employing the primers and amplification conditions proposed by Scheutz et al. [Reference Scheutz17].
Identification of typical and atypical EPEC/EAEC strains and ETEC ST toxin gene (st) variants
Strains presenting eae and aatA genetic markers were further investigated for bfp and aggR genes (Table 1) defining typical EPEC and EAEC, respectively. Strains negative for these genes were classified as atypical EPEC/EAEC. ST toxin gene (st)-positive ETEC strains were submitted to an additional duplex PCR (Table 1) in order to investigate the presence of human and porcine variants.
Phenotypic differentiation between EIEC and Shigella strains
Given that ipaH genetic marker can be present in both EIEC and Shigella, and the possible occurrence of cross-reactivity among serogroups of these two bacteria in serological tests, all the strains positive for ipaH gene in PCR assays were submitted to an extended biochemical profiling [Reference Ewing18]. Only strains positive for citrate, mucate and sodium acetate utilization were considered as EIEC.
Serotyping
Strains classified in any of the DEC pathotypes investigated by PCR were O:H serotyped by tube agglutination assays [Reference Ewing18] employing absorbed somatic (O1-O188) and flagellar antisera (H1-H56) produced at IAL. Non-motile ETEC strains of serogroup O6 were subjected to PCR-RFLP in order to identify the allelic forms of their fliC genes [Reference Machado, Grimont and Grimont19].
Cytotoxicity assays
Strains harbouring stx genes were confirmed as STEC in cytotoxic assays employing cultured Vero cells [Reference Konowalchuk, Speirs and Stavric20].
Statistical analyses
The χ 2 test was employed to test the hypothesis that the distribution of each pathotype was not homogeneous among the distinct age groups of patients. The analysis was performed with SAS 9.3 (SAS Institute, Cary, NC). P-value of <0.05 was considered to indicate statistically significant differences.
Results and discussion
DEC strains are considered major aetiological agents of diarrhoeal diseases in Brazil, and worldwide [Reference Kotloff1, Reference Moreno10, Reference Bueris21]. Nevertheless, updated information on DEC circulation in Brazilian settings is not currently available. Patterns in the circulation of diarrhoeagenic pathotypes and serotypes tend to change over time and may vary between different countries. Therefore, the primary aim of this study was to describe the occurrence of pathotypes and serotypes of DEC isolated from sporadic and outbreak cases of acute diarrhoea and HUS, during a period of 6 years of active epidemiological surveillance, performed in different Brazilian states. However, among diarrhoeagenic eae-harbouring E. coli-like colonies, we identified 10 E. albertii isolates, and the objective of this study was extended to encompass the analysis of such isolates.
A total of 693 (13.7%) cases were positive for DEC or E. albertii. DEC strains representing one of the five major pathotypes were detected as the sole enteric pathogen in 683 (13.5%) cases. E. albertii could be found in 10 (0.2%) of the total cases. The frequency of DEC strains reported in the present study is similar to previously reported data for China and Nigeria [Reference Huang22, Reference Ifeanyi23], but lower than that reported in Mexico [Reference Canizalez-Roman24]. However, the real prevalence of DEC in Brazil could be greater, since in a large number of diarrhoeal cases reported, including outbreaks, the aetiologic agents are not identified due to insufficient epidemiological investigation or technical limitations. The reliable classification of DEC into distinct pathotypes requires the use of molecular tools. Since many local public health laboratories in Brazil are not adequately equipped to perform molecular techniques, most DEC infections are probably missed.
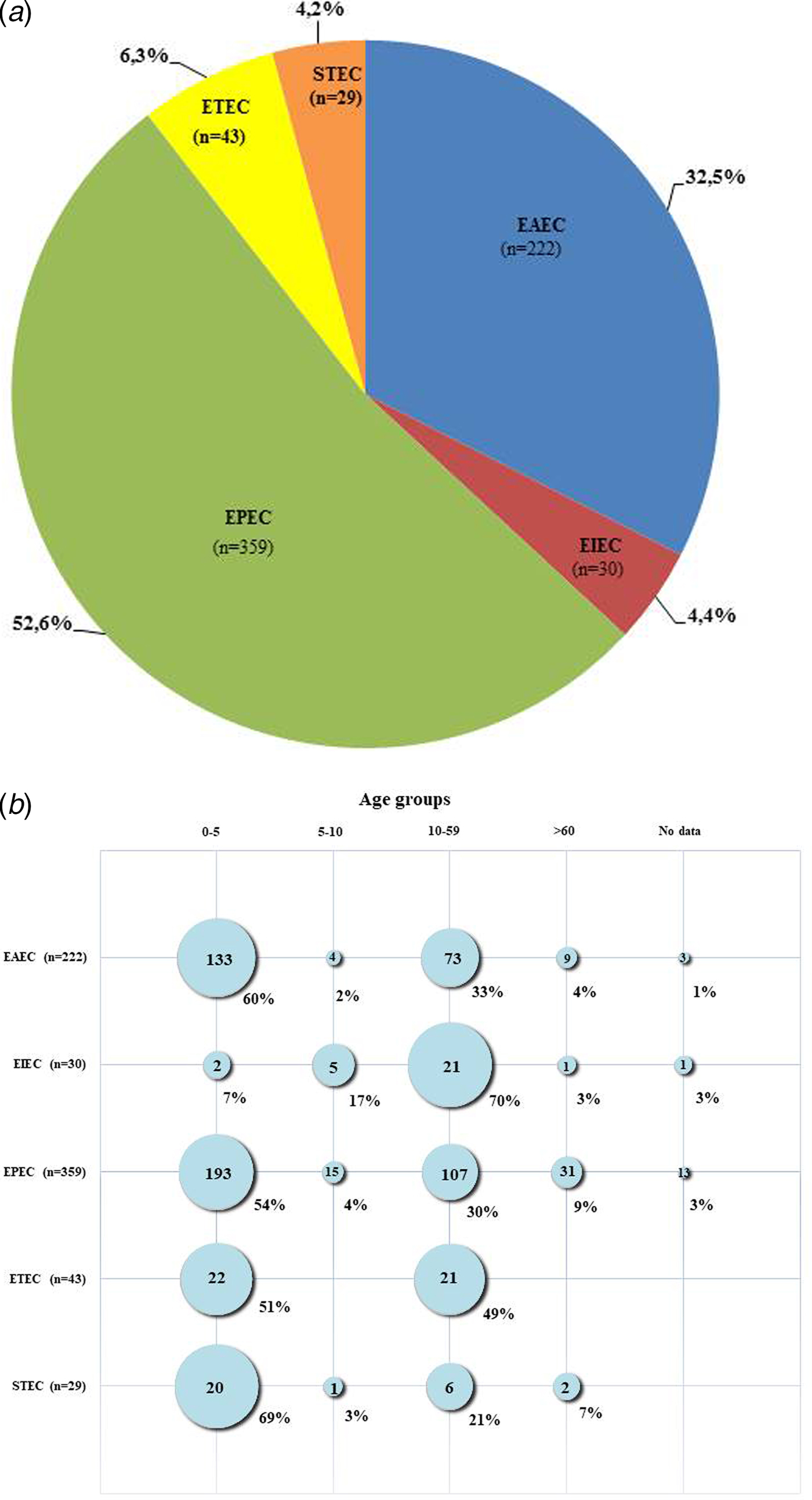
Figure 1a shows the distribution of the different DEC pathotypes among positive DEC strains in this study. The most frequent pathotype was EPEC, found in 359 (52.6%) of the positive DEC cases, followed by EAEC present in 222 (32.5%) of the cases. ETEC, EIEC and STEC were identified in 43 (6.3%), 30 (4.4%) and 29 (4.2%) of the positive cases, respectively. By comparing current results with studies conducted earlier in Brazil, two important differences were noticed: in prior years, EAEC strains were found to be more frequent than EPEC [Reference Moreno10, Reference Bueris21], but presently, the occurrence of EPEC was higher than EAEC. In addition, according to previous reports, STEC and EIEC pathotypes were not found or were rarely diagnosed in cases of diarrhoea [Reference Moreno10]. In this study, however, both pathotypes were found, albeit at lower frequencies compared with EPEC and EAEC. Our findings support the suggestion that there was a shift in the pattern of circulation of EAEC and EPEC strains in Brazil in recent years. Previously, EAEC were most common but EPEC have become predominant. However, the differences between the results of this study and earlier Brazilian studies may be due to the focus of earlier studies on specific regions and on children under 5 years of age [Reference Moreno10]. So, the data they provided regarding the circulation of DEC pathotypes although useful could have been biased by local factors.

Fig. 1. (a) Pathotypes among 683 cases of enteric infection caused by diarrhoeagenic Escherichia coli (DEC) in Brazil during the years of 2011–2016. (b) Occurrence of pathotypes in different age groups of patients affected by DEC strains in Brazil during the years of 2011–2016.
Figure 1b shows the age distribution for all DEC-positive samples collected from Brazilian patients ranging from 3 months to 96 years old. Most (370; 54%) of the DEC strains were isolated from individuals aged up to 5 years old. However, an analysis of the occurrence of the different pathotypes by age groups showed some differences regarding individual pathotypes. Most EPEC, EAEC and STEC strains were isolated from patients aged <5 years old, whereas most EIEC strains occurred among those aged >10 years old (22; 73%). ETEC infections occurred almost equally in children and adults, being found in 22 (51%) of cases involving children younger than 5 years old and in 21 (49%) cases of individuals older than 10. This pathotype did not occur in subjects older than 60. Statistical analyses demonstrated that DEC pathotypes were not equally distributed among the distinct age categories (P < 0.001), with the exception of ETEC, which was equally distributed between the two age groups from which this pathotype was isolated (P > 0.05). Enteric infections affecting young children may have serious negative consequences, so in most studies [Reference Kotloff1], including studies performed in Brazil [Reference Moreno10, Reference Bueris21], this population is preferentially targeted. There is evidence that frequent and persistent infections due to DEC can lead to impairments in physical and cognitive development [Reference Guerrant25]. Moreover, age is a risk factor for HUS development after STEC infections, and children <5 years old are considered to be at greater risk [Reference Proulx, Seidman and Karpman26]. The two laboratory-confirmed HUS cases in this study involved patients aged <5 years. Therefore, considering that infectious diarrhoea more often affects young children, and can also be more detrimental to them, we advise that this group of patients must receive priority for diagnosis and intervention measures.
All the identified EPEC strains of this study, except one, were classified as atypical (aEPEC), as they lacked bfp gene. The only typical (tEPEC) we found was a strain belonging to serotype O157:H39, isolated in 2011 from a child. Since the original description of EPEC in the middle of 1940s [Reference Bray27], tEPEC was the leading cause of childhood diarrhoea. However, in the 1990s, for undetermined reasons, a decline in the incidence of tEPEC was observed worldwide with concomitant rise in the incidence of aEPEC [Reference Ochoa28, Reference Rodrigues29], which is nowadays by far more prevalent than tEPEC in many locations. This trend has also been observed in Brazil [Reference Rodrigues29], however, care should be taken in analysing this phenomena, as previously the identification of EPEC was based solely in serogroup determination and the presence of bfp gene was not routinely sought. Atypical EPEC infections affect both children and adults, and have been linked to acute, persistent and outbreaks of diarrhoeal diseases in several countries, including Brazil [Reference Vieira12].
Among the aEPEC strains of this study, 86 serogroups were identified and their association with distinct flagellar antigens resulted in 96 different serotypes. The diversity of serogroups and serotypes found among aEPEC strains in our study is shown in Table 2. As it can be noted, no predominant serotype was identified in the period analysed, although some specific ones like O126:H19 and O33:H34 were found more often than the others. Moreover, we also observed the presence of serotypes as O145:HNM, O55:H7, O63:H6 and O26:H11/HNM that are frequently associated with STEC pathotype, raising the speculation that these aEPEC could actually represent strains that were originally STEC before loss of stx genes, which are bacteriophage-borne [Reference Bielaszewska30]. There were serotypes such as O39:HNM that have already been linked with EPEC diarrhoeal outbreaks [Reference Hedberg31]. Serotype O2:H16 in particular has been reported as an agent of aEPEC outbreaks in Brazil [Reference Vieira12]. The finding of a great diversity of serotypes among aEPEC in this study is in agreement with other studies [Reference Tennant32], demonstrating the heterogeneous nature of aEPEC in terms of antigenic and virulence features. It has been suggested that not all aEPEC strains are in fact pathogenic and many human subjects can be asymptomatic carriers of the bacteria [Reference Hernandes4]. Currently, we cannot determine whether all the aEPEC serotypes circulating in our settings are indeed relevant in clinical and epidemiological terms. However, it is important to continue monitoring aEPEC strains and to employ whole genome sequencing approaches to uncover the most virulent and potentially epidemic clones in order to clarify questions regarding aEPEC virulence potential.
Table 2. O:H antigenic types (serotypes) found among 683 DEC strains isolated between 2011 and 2016 in Brazil

OR, O rough; ONT, O non-typeable; HNM, H non-motile; HNT, H non-typeable.
a Typical EPEC (bfp+).
In our analysis, strains carrying aggR gene, thus classified as typical EAEC, were 84% (187/222) of the total of EAEC-positive strains, while 16% (35/222) of the EAEC in this study were atypical and lacked the gene. These findings are similar to previous reports that the majority of the EAEC strains linked to enteric illness harbour aggR [Reference Cennimo33]. As the aggR gene product regulates the expression of most of the currently identified virulence factors of EAEC, in some studies, it is suggested that only typical EAEC are pathogenic for humans [Reference Estrada-Garcia and Navarro-Garcia3]. However, there has been a report implicating atypical EAEC with diarrhoeal cases [Reference Itoh34]. The fact that in this study we did not find any other bacterial or viral enteropathogens in samples positive for atypical EAEC corroborates this previous report and is evidence for a role for atypical EAEC in enteric illness.
EAEC strains in this study included 42 distinct O:H serotypes, 35 of them associated with typical isolates and 12 associated with atypical EAEC (Table 2). Serotypes O11:H18, O15:H2, O175:H28, O38:H25, O73:H18 were common to both typical and atypical strains, and one can speculate that these aEAEC belonging to these serotypes of typical EAEC could have lost aggR-bearing plasmid. Serotypes O175:H28, O15:H2 and O153:H2 were more commonly found among typical EAEC. However, the majority of the EAEC strains of this study, irrespective of the fact they were typical or atypical, could not have their O antigens determined due to roughness or absence of antisera reactivity. The self-agglutinating nature of EAEC strains together with serotype diversity [Reference Weintraub35, Reference Jenkins36] limits the use of serological techniques in outbreaks tracing and laboratory surveillance. Nevertheless, some interesting findings regarding antigenic features of EAEC of this study could be observed. The H2 antigen was frequently associated with typical EAEC. Some of the serotypes found in this study have been reported in other studies conducted in Brazil, but serotypes O175:H28 and O15:H2 were not previously reported, though they were the most common serotypes found in the period of this study [Reference Regua-Mangia37, Reference Uber38]. Strains of serogroup O15 with H18 antigen had been reported previously in Brazil as atypical EAEC [Reference Regua-Mangia37]. In this study, O15 serogroup was found in combination with H2 flagellar type and mostly among typical EAEC.
ETEC is a major cause of moderate-to-severe diarrhoea in developing countries especially in Asia [Reference Qadri39].This pathotype is also an important enteric pathogen in South America and earlier studies in Brazil have reported ETEC infections, including outbreaks, in different regions [Reference Moreno10, Reference Bueris21, Reference Vicente40]. In the present study, ETEC was isolated in 43 (6.3%) of the total of diarrhoeal cases analysed. Our results confirm that although less frequent than other DEC pathotypes such as EPEC and EAEC, ETEC are still responsible for causing enteric illness in our country, and must therefore continue to be considered in the list of enteric pathogens to be sought for the diagnosis of diarrhoeal diseases. In this study, we found the profile lt+/st+ as the most common toxigenic genotype among ETEC-positive isolates, being present in 56% (24/43) of these strains, while 44% (19/43) of the strains harboured only LT enterotoxin-related gene lt. None of the strains studied carried st gene alone. It has been reported that st or st/lt carrying ETEC strains, rather than lt only harbouring ETEC, are more often associated with moderate-to-severe diarrhoea and a higher risk of death in young children [Reference Kotloff1]. In the st+ isolates, st h gene variant was carried by 21 strains, while only one strain possessed the st p variant. ST enterotoxin variants STh and STp can both induce diarrhoea in humans; however, the human variant is considered to be more relevant in clinical terms due to its higher prevalence when compared with STp [Reference Fleckenstein41]. Three ETEC strains did not give any result in relation to the st gene variants searched. No information regarding the toxigenic profiles of ETEC isolated previously in Brazil is available; therefore, our findings although derived from a small number of strains are the only data available on this topic. Examination of the 43 ETEC isolates for O:H antigens demonstrated the occurrence of 14 distinct serotypes (Table 2). However, 25 (58%) of these strains belonged to the single serotype O6:H16, including 22 motile strains (H16 antigens expressed) and three non-motile strains (H16 type was determined by PCR-RFPL analysis of fliC genes). ETEC of serotype O6:H16 is of worldwide occurrence, being one of the most common serotypes associated with ETEC infections in humans [Reference Croxen2].
All the strains positive for stx genes in this study were phenotypically confirmed as STEC in Vero cell cytotoxic assays. We had 19 strains (65.5%) carrying stx1, while nine (31%) carried only stx2 and one strain carried both stx1 and stx2 (Table 3). Previous studies characterizing STEC of clinical origin in Brazil have also reported that most of the isolates harboured only stx1 and were from cases of acute non-complicated diarrhoea [Reference Vaz42]. Subtyping of stx genes revealed that stx1a allele was carried by all the stx1-positive strains, except one that had stx1d (Table 3). In relation to stx2 subtypes, we encountered the allele 2a in association with 2c, 2d or 2e in five of the stx2-positive STEC, while subtypes 2c and 2e were found alone in four strains (Table 3). Subtypes 2b and 2g were not present. Stx2 and subtypes 2a, 2c and 2d are more often linked with complicated STEC infections and their association with some specific O serogroups and adherence factors can be a predictor of greater probability of HUS [Reference Usein43–Reference Zhang45]. In this study, we were able to demonstrate that these three most problematic stx2 subtypes were found in most of stx2-positive strains. However, STEC strains producing Stx1 can also cause HUS [Reference Zhang45], so the possibility that stx1a-bearing Brazilian isolates can be responsible for more complicated infections exist and for this reason they must be carefully monitored.
Table 3. Serotypes and stx genotypes among 29 STEC strains recovered from human infections in Brazil from 2011 to 2016
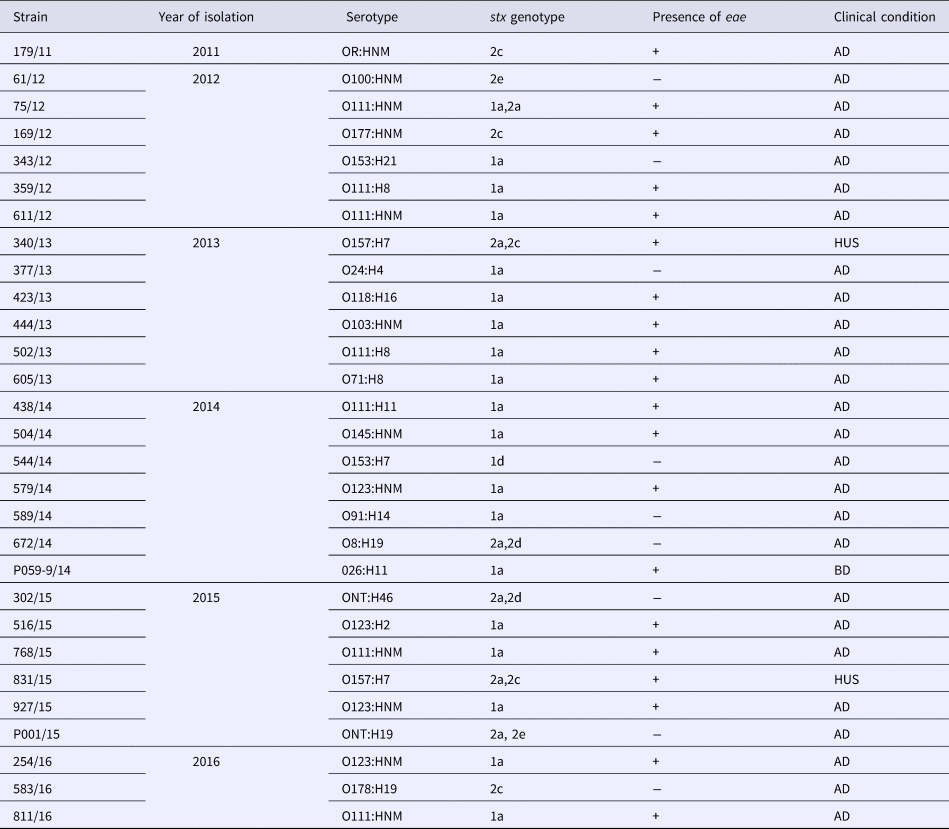
AD, acute diarrhoea; BD, bloody diarrhoea; HUS, haemolytic uremic syndrome.
Twenty (69%) of the STEC strains herein analysed possessed eae gene, while nine strains (31%) lacked this marker (Table 3). This indicates that the majority of the human STEC infections in Brazil, in the period covering this study, were caused by strains dotted with the potential ability to express both Shiga toxin and A/E phenotypes. The potential to form A/E lesions by STEC isolates is regarded as an additional risk factor in the clinical outcome of STEC diseases, as there is a higher risk of HUS development [Reference Paton and Paton9]. In fact, most of the HUS cases registered in Brazil [Reference Souza46] including the two cases analysed in this study were caused by strains carrying stx2 and eae. In this study, STEC strains fell into 15 distinct serotypes (Table 2), and included serotypes of major epidemiological importance such as O157:H7, O111:H8/NM, O26:H11, O145:HNM, O103:HNM, as well as serotypes which have been implicated in human disease, but isolated less frequently [Reference Saupe47]. The most frequent serotype presently observed was O111:H8. The same situation was observed in prior years in Brazil where STEC O111 was the most frequent serogroup encountered in human diseases [Reference Vaz42]. By comparing the present results with data about STEC serotypes in Brazil spanning the period of 1979–2004, we could note that the diversity of serotypes detected in this study was greater than that observed before. This may be indicative of the efforts that have been made in Brazil to increase the detection of STEC pathogens by employing molecular approaches targeting stx genes, instead of serogroup-based screening by immunological methods, which were largely performed in the past. This change in methodology must continue and should be implemented in the largest possible number of laboratories in Brazil, for the benefit of future surveys addressing STEC infection epidemiology.
Studies describing the occurrence and markers for EIEC circulation are scarce. This is due to the fact that EIEC differentiation from Shigella is often problematic as these two bacteria are closely related and almost identical in terms of genetic contents. Additionally, surface antigens of EIEC and Shigella cross-react, so serological tests may not be a suitable option. In fact, there is evidence from phylogenetic studies demonstrating that EIEC strains represent intermediate forms in the evolution from commensal E. coli to Shigella [Reference Peng, Yang and Jin48]. Differentiation among EIEC and Shigella is possible only through extensive biochemical profiling which can be performed solely in reference laboratories. As a consequence, EIEC strains are under-represented in most epidemiological surveys. Therefore, EIEC contribution to the burden of DEC infections is largely overlooked. In this study, phenotypically confirmed EIEC corresponded to 4.4% of DEC strains, showing that this pathotype has a role in community-acquired diarrhoea in Brazil. Serogroups O132, O121 and O124 were the most common, being present in 9/30 (30%), 7/30 (23%) and 6/30 (20%) of strains, respectively, and serotype O132:H21 was the most frequent. Serogroups O121 and O124 are among the most commonly reported among EIEC strains [Reference Croxen2], and in this sense, our results only partially agree with previous reports, as in this study the O132 serogroup was the most prevalent. EIEC outbreaks have been reported in other countries [Reference Newitt49] and we are currently performing PFGE typing to assess the genetic relatedness among strains of the same serotype isolated in this study.
Ten of the eae-harbouring strains, which had been previously identified in our routine laboratory testing as EPEC, were actually found to be E. albertii. The recognition of E. albertii is challenging in that with few exceptions their biochemical profile and most of the virulence markers resemble DEC pathotypes EPEC and STEC. Only recently genomic approaches have allowed accurate discrimination, reallocating these strains to another taxonomic position [Reference Ooka50]. This is the first report on the occurrence of E. albertii from active surveillance of foodborne diseases in Brazil. The majority of the E. albertii were untypeable or rough regarding their somatic antigens, and were non-motile, so their O:H serotypes could not be identified. This is in agreement with previous reports of the antigenic untypeability of E. albertii strains [Reference Nimri51]. It is worth mentioning that there is no specific typing scheme for E. albertii and attempts to determine their somatic and flagellar antigens usually employ antisera produced against E. coli strains. This suggests that E. albertii O and H antigens may have distinct characteristics in relation to E. coli antigens. However, there were two exceptions in this study, as two strains reacted with O113 E. coli antisera, but were non-motile, rendering serotype O113:HNM. One of the analysed E. albertii strains in this study was positive for stx2f gene. Production of Stx2f by E. albertii strains has been reported [Reference Ooka7] and one can speculate about the potential of these strains to cause more serious diseases. So far, E. albertii appears to represent a small proportion of the diarrhoeagenic strains circulating in Brazilian settings, but even so they must receive attention in surveillance programmes in Brazil and elsewhere, so that it will be possible to determine how their circulation trends will evolve.
We attempted to draw a scenario for DEC strains occurrence in comparison to prior years in Brazil, and with data from other countries. Although several problems were faced, especially related to logistical difficulties in sending bacterial isolates to reference laboratories for analysis, we believe the present study contributes a useful ‘snapshot’ on the aetiology of diarrhoeal diseases caused by DEC strains in our country. Certainly this will be very important for future studies and considering intervention measures. The continuous epidemiological surveillance of food and water transmissible diseases and characterization of DEC strains associated with human infections is essential for the recognition of new patterns of pathogen virulence and circulation. In this regard, it is of paramount importance that public health and clinical laboratories involved in infectious diseases diagnosis and surveillance are capable of correctly recognizing DEC strains.
Acknowledgements
The authors are grateful to Professor J. C. F. Pantoja for assistance with statistical analysis. The authors also acknowledge the work of all the professionals of the State Public Health Laboratories in Brazil involved in the laboratory surveillance of enteric diseases.
Conflict of interest
None.