Article contents
LiMn2O4 microspheres secondary structure of nanoparticles/plates as cathodes for Li-ion batteries
Published online by Cambridge University Press: 23 April 2013
Abstract
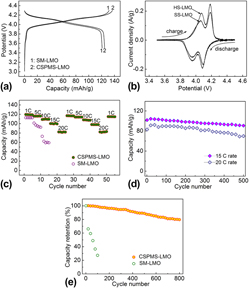
In this work, we succeeded in synthesis of spinel LiMn2O4 via a facile self-template method. The product displays a micro-/nanohybrid structure. Nanoparticles/plates act as the primary nanoblocks to build the secondary microarchitecture. There is the open space between the nanoblocks and the void space between the secondary structures. Electrochemical tests demonstrate that the as-synthesized sample exhibits superior rate capability and high-rate cycleability when contrasted with its solid counterpart. The initial discharge capacity is 126 mAh/g at 0.1 C, 110 mAh/g at 10 C, and 84 mAh/g at 20 C. The discharge capacity retention of about 80% is obtained after 800 cycles at 10 C. The high capacity and excellent cycling life of the material shows its potential for application as high-power batteries. The improved rate capability and cycleability can be attributed to its secondary structure that can facilitate fast Li-insertion/extraction and buffer the volume expansion/contraction upon cycling.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 7
- Cited by




