Introduction
Understanding a species’ habitat requirements can be key to its conservation, helping to improve assessments of the status and threats to populations and inform appropriate management strategies (Sutherland et al. Reference Sutherland, Newton and Green2004, Martin et al. Reference Martin, Perrin, Boyes, Abebe, Annorbah, Asamoah, Bizimana, Bobo, Bunbury, Brouwer, Diop, Ewnetu, Fotso, Garteh, Hall, Holbech, Madindou, Maisels, Mokoko, Mulwa, Reuleaux, Symes, Tamungang, Taylor, Valle, Waltert and Wondafrash2014). However, for many species, gaps exist in this vital information (Brooks et al. Reference Brooks, Collar, Green, Marsden and Pain2008). Parrots, for example, are amongst the most globally threatened groups of birds but little is known about the habitat requirements of the majority of parrots in Africa (Martin et al. Reference Martin, Perrin, Boyes, Abebe, Annorbah, Asamoah, Bizimana, Bobo, Bunbury, Brouwer, Diop, Ewnetu, Fotso, Garteh, Hall, Holbech, Madindou, Maisels, Mokoko, Mulwa, Reuleaux, Symes, Tamungang, Taylor, Valle, Waltert and Wondafrash2014, Olah 2016). Despite concerns over the impact of habitat loss on several species in Africa, there have been few attempts to systematically assess the status and threats to populations (Snyder et al. Reference Snyder, McGowan, Gilardi and Grajal2000, Martin et al. Reference Martin, Perrin, Boyes, Abebe, Annorbah, Asamoah, Bizimana, Bobo, Bunbury, Brouwer, Diop, Ewnetu, Fotso, Garteh, Hall, Holbech, Madindou, Maisels, Mokoko, Mulwa, Reuleaux, Symes, Tamungang, Taylor, Valle, Waltert and Wondafrash2014, Mzumara et al. Reference Mzumara, Perrin and Downs2014).
Lilian’s (or Nyasa) Lovebirds Agapornis lilianae have a restricted range within the Zambezi River basin centred on Zambia, with additional populations occurring in northern Zimbabwe, southern Malawi and western Mozambique (Hockey et al. Reference Hockey, Dean and Ryan2005). Local extinctions and population declines have been reported in several parts of their range and concerns over their conservation status led to their classification as ‘Near Threatened’ on the IUCN Red List (BirdLife International 2016).
Lilian’s Lovebirds are generally recognised as having a close association with mopane Colophospermum mopane woodland (Forshaw Reference Forshaw1989, Collar Reference Collar, del Hoyo, Elliott and Sargatal1997), however they are notably absent from some areas of apparently suitable habitat (Forshaw Reference Forshaw1989), suggesting that particular characteristics of mopane woodland could be important for the species. Large areas of these woodland habitats within the species range have been modified between 1972 and 1989 in Zambia (Yang and Prince Reference Yang and Prince2000). This is partly due to activities such as charcoal and timber production which are significant economic activities in mopane woodlands (Malimbwi et al. Reference Malimbwi, Chidumayo, Zahabu, Kingazi, Misana, Luoga, Nduwamungu, Chidumayo and Gumbo2010, Woollen et al. Reference Woollen, Ryan, Baumert, Vollmer, Grundy, Fisher and Lisboa2016). This raises the possibility that habitat degradation could be a threat to the species.
An assessment of the status of, and threats to, the global population of Lilian’s Lovebirds has been identified as a priority action for this species (Perrin Reference Perrin2012, Martin et al. Reference Martin, Perrin, Boyes, Abebe, Annorbah, Asamoah, Bizimana, Bobo, Bunbury, Brouwer, Diop, Ewnetu, Fotso, Garteh, Hall, Holbech, Madindou, Maisels, Mokoko, Mulwa, Reuleaux, Symes, Tamungang, Taylor, Valle, Waltert and Wondafrash2014, BirdLife International 2016). Studies previously conducted in Malawi suggested that naturally occurring tree cavities, used for nesting and breeding, are a key resource for the species and that the distribution of mature trees may be an important driver of occurrence (Mzumara et al. Reference Mzumara, Perrin and Downs2016a). In this study we aimed to understand the habitat associations of Lilian’s Lovebird in Zambia, and specifically to determine the structural components of mopane woodlands that may be critical for this poorly known small parrot species.
Methods
Survey area
In order to systematically select sites to survey in the field, we initially built a basic species distribution model using the maximum entropy approach, MaxEnt (version 3.3.3k; http://www.cs.princeton.edu/∼schapire/maxent/; Phillips et al. Reference Phillips, Dudik and Schapire2004, Reference Phillips, Anderson and Schapire2006). This model was based on historic records of Lilian’s Lovebirds sightings from Zimbabwe, Malawi and Zambia. These records were obtained from the following sources: Dowsett-Lemaire and Dowsett (Reference Dowsett-Lemaire and Dowsett2006), Dowsett et al. (Reference Dowsett, Aspinwall and Dowsett-Lemaire2008), Global Biodiversity Information Facility (GBIF) database, WorldBirds Database and from independent bird watchers who had recorded the species on their birding trips. Variables used to build the MaxEnt model were climate and vegetation data. Precipitation and temperature data were obtained from the WorldClim dataset (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005), whilst a vegetation map was obtained from the WWF’s Terrestrial Ecoregions of the World (TEOW) (Olson et al. Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001). The model output identified areas, at a resolution of 1 km2, predicted to be suitable for the species and provided a probability of occurrence for each square. MaxEnt has its biases and is known to overestimate occurrences on the fringes of a species’ range (Yackulic et al. Reference Yackulic, Chandler, Zipkin, Royle, Nichols, Campbell Grant and Veran2013) thus it is important to validate its outputs. We therefore randomly selected 200 sampling points from the areas predicted to be occupied by lovebirds (Figure 1). These points were randomly selected using ArcGIS Version 10.2.1. (ESRI 2014). A subset of 116 of these points were then visited based on logistical considerations; primarily the difficulty of accessing points located in very remote locations. Detailed assessments of woodland structure were made in a subset of 54 of these points which contained mopane woodland, which for the purposes of this study was defined as all the points which contained at least one Mopane tree greater than 0.1 m diameter at breast height (DBH).
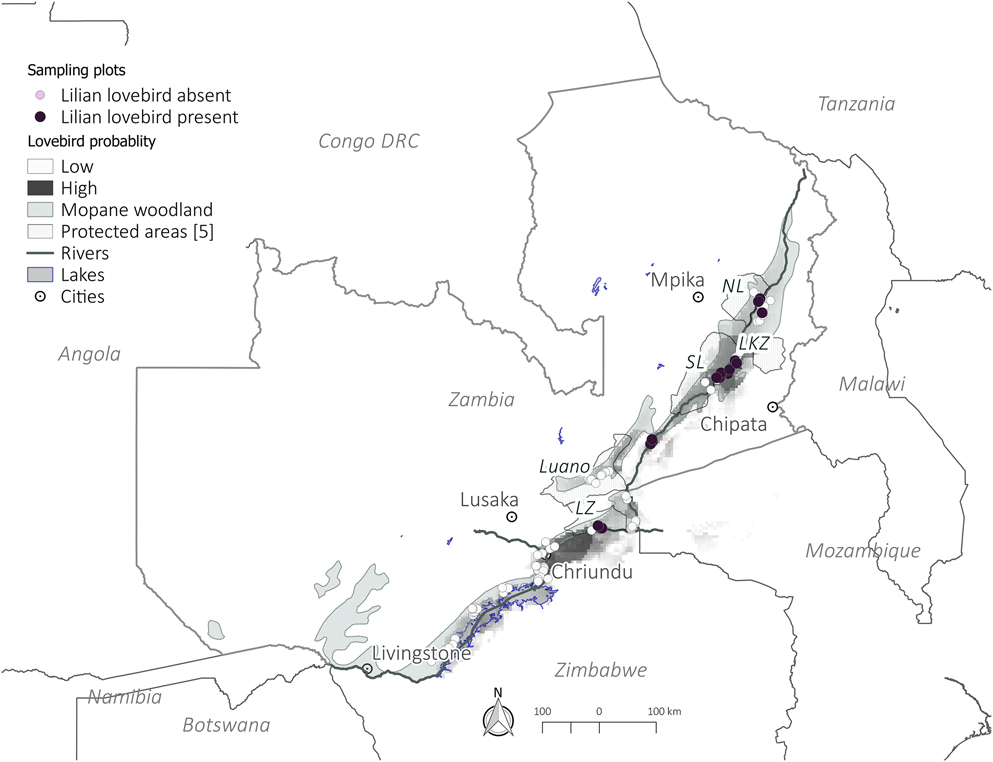
Figure 1. Occurrence of Lilian’s Lovebirds in Zambia. Shaded areas indicate the MaxEnt model showing areas of high and low probability of occurence of Lilian’s Lovebirds used to select points to be surveyed. LZ - Lower Zambezi, SL - South Luangwa NP, NL - North Luangwa NP, LKZ - Lukusuzi NP.
Sampling focused on the Zambezi (11°22′11″S, 24°18′30″E), Luano (14°49S, 29°34E) and Luangwa (15°34′27.95″S, 30°23′19.93″E) valleys (Figure 1). The Luangwa and Zambezi Valleys are historically known as the strongholds of Lilian’s Lovebird’s global range (Dowsett et al. Reference Dowsett, Aspinwall and Dowsett-Lemaire2008). However, there are no previous confirmed sightings of Lilian’s Lovebirds from the Luano valley, which could be because of its difficult access (Dowsett et al. Reference Dowsett, Aspinwall and Dowsett-Lemaire2008). Due to the likely presence of suitable habitat and proximity to populations in the Luangwa valley it has been speculated that the Luano Valley could contain populations of Lilian’s Lovebird (Dowsett et al. Reference Dowsett, Aspinwall and Dowsett-Lemaire2008). The three valleys cover an area of approximately 46,965 km2.
Field surveys were conducted at the beginning of the dry season (May–July) in 2015. We selected this period to ensure access to our areas of interest as flood waters would have receded by then. This time was also selected based on knowledge of the species’ ecology from studies in Malawi (Mzumara Reference Mzumara2015, Mzumara et al. Reference Mzumara, Perrin and Downs2016a). It is expected that during this period, lovebirds would have just completed their breeding season and be foraging very close to their breeding areas (Mzumara Reference Mzumara2015). This was important for our method of sampling as during the non-breeding season the lovebirds may forage very far from their known roost/breeding areas (i.e. mopane woodlands), thus detection is low (Mzumara et al. Reference Mzumara, Perrin and Downs2018).
Lilian’s Lovebird surveys
Each sampling site was a square of 1 km x 1 km. This scale was selected based on our understanding of the spatial ecology of Lilian’s and other lovebird species (Mzumara et al. Reference Mzumara, Perrin and Downs2016b, Ndithia and Perrin Reference Ndithia and Perrin2006). At each site, two transects, each 1 km in length were identified, running parallel to each other, 500 m apart. The start and end points of each transect were 250m from the corner of the square (Figure S1 in the online Supplementary Material). Transects were walked at approximately 1 km hr-1. We recorded the presence of all Lilian’s Lovebirds seen or heard during the transect walk.
Woodland structure surveys
Measurements of woodland structure were made in four circular plots within each sampling site, two along each 1-km transect, 250 m from the start and endpoint. As the transects were 500 m apart this meant that the four plots were effectively corners of a square 500 m x 500 m (Figure S1). Each circular plot had a radius of 15 m within which measurements of tree size and tree community composition were made. A ‘tree’ was defined as any stem which had a DBH of at least 0.1 m (DBH measurements were taken at 1.3 m above the ground to ensure uniformity).
In each circular plot we recorded the total number of mopane and other tree species. Tree height was measured using a Nikon Laser Rangefinder Forestry Pro (Nikon Vision Co. Ltd, Tokyo, Japan), and a diameter tape (Lufkin W606PD 1/4-Inch by 6-Foot Diameter Tape) was used to measure DBH. The distance of each tree to the nearest tree was recorded using a tape measure. The mean and maximum of each of the variables (DBH, height, no. of mopane, no. of other trees), were calculated separately for each of the four circular plots within each quadrat. Aggregate measures for each sampling square were calculated from the means and maxima of the four circular plots. Elevation was recorded for each plot as Forshaw (Reference Forshaw1989) suggested that the species occupies only a restricted range of altitudes. Elevation values were measured using a handheld GPS.
We used a principal component analysis (PCA) on data across all sampling sites. The nine variables included in the PCA are shown in Table S1 in the online supplementary materials. The first three principal components (axes) explained about 90% of the variation in the data. The first axis (eigenvalue = 1.84) explained 42% of the variation and was positively correlated with mean DBH and mean height of trees. Hereafter we refer to this variable as PC1Size. The second axis (eigenvalues =1.53) explained 29% of the variation, was closely related to the density of Mopane trees and will be referred as PC2Mopane. The third axis (eigenvalue of 1.11) explained 15% of variation, was also closely related to the density of other tree species and hereafter referred to as PC3Other. This approach follows that of Lewis et al. (Reference Lewis, Amar, Taylor, Grice and Smith2009) who explored the stand structure of another potential habitat specialist.
As DBH is a commonly used measure of tree size by forestry managers and conservation practitioners, and because it was found to be the measure of tree size most closely associated with PC1Size (Figure S2) we further defined the relationship between DBH and lovebird occurrence with a view of developing a practical tool for the management of Mopane woodland for Lilian’s Lovebirds.
Statistical analysis
A general linear model (GLM) with a binomial error structure and a logit link-function was used to explore the association between the presence of Lilian’s Lovebirds, the three habitat variables (PC1Size, PC2Mopane, PC3Other) and elevation in metres above sea level (Table S1). Lilian’s Lovebird presence (1) or absence (0) was the two-factor binary response variable and each of the explanatory variables were fitted as main effects. The global model included all four variables and all nested models were considered as alternative candidate models. We conducted model selection using Akaike’s Information Criterion corrected for small sample size (AICc) and multi-Model inference with the MuMin package (Bartoń Reference Bartoń2013) to determine which variables were associated with the presence of Lilian’s Lovebirds. Models were ranked using AICc values and the top models selected as all of those within two AICc units of the model with the lowest AICc, which was considered to be the optimal model. Akaike weights were estimated for each model (wi) following Burnham and Anderson (Reference Burnham and Anderson2003). We assessed the relative importance of our different covariates by summing the wi of each model in which the variable appeared for all plausible models (Di, 4). We also used this model subset to generate parameter estimates and their 95% confidence limits through model averaging.
To check the performance of the optimal model and its overall ability to predict the presence of Lilian’s Lovebirds correctly, we calculated the area under curve (AUC). The AUC is considered a numerical index used to summarise the Receiver Operating Characteristics (ROC; Hanley and McNeil Reference Hanley and McNeil1982). AUC values between 0.5 and 0.7 suggest that the model has a low accuracy. Whilst those between 0.7 and 0.9 have moderate accuracy (Streiner and Cairney Reference Streiner and Cairney2007). We used the package ROCR (Sing et al. Reference Sing, Sander, Beerenwinkel and Lengauer2005) to calculate the ROC of the optimal model. Low AUC suggests that factors other than those included in the model also influence the response variable. All analyses were conducted using R v.3.2.2 (R Core Team 2013).
Results
Of the 116 sample sites selected a priori, the majority of points (53%) lacked the targeted mopane-woodland habitat (mopane with DBH of 0.1m and above). In some instances this was due to human disturbance (i.e. charcoal-making, clear-cutting of woodland for conversion to agriculture or overgrazing by livestock) while in others the habitat was dominated by non-mopane vegetation or only contained small, scrub-type mopane trees. Lilian’s Lovebirds were not observed in any of the non-mopane-woodland sites. Our analysis was restricted to the 54 sites in which measurements of mopane woodland structure could be made. Of these, Lilian’s Lovebirds were recorded in 20 sites (Appendix S5). The number of lovebirds seen in a square ranged from two to 50+ individuals. No lovebirds were recorded in any areas west of Lower Zambezi National Park nor in the Luano valley (Figure 1).
Influence of vegetation variables on presence of Lilian’s Lovebirds
The best performing models (optimal plus two top models with ∆AICc < 2 from the optimal model) from our analysis (Table S3) featured three variables; PC1Size, PC2Mopane and PC3Other. The optimal model contained PC1Size alone and this variable featured in all the top models (Table S2) meaning it had relative importance score of 1. When parameter estimates were averaged across the top three models, PC1Size was the only variable with 95% confidence intervals that did not overlap zero. The other two variables, PC2Mopane and PC3Other each featured in one of the two other best models and thus had low relative importance scores of 0.27 and 0.24, respectively. Furthermore, confidence intervals of the parameter estimates for these variables overlapped zero, suggesting a relatively weak effect on lovebird occurrence (Table S3). The AUC for our top model (PC1Size) was 0.82 indicating that our model had moderate accuracy.
The DBH measurement showed the most influence on the PC1Size (Figures 2 and S2; Table S2). The majority (75%) of sightings of Lilian’s Lovebirds occurred in areas of woodland where mean DBH was greater than 0.3 m. In addition, lovebirds were seen in all sites (n = 11) where mean DBH was greater than 0.4 m. Our model predicted that in sites where the mean DBH of mopane was greater than 0.35 m, there was > 50% probability of the site being occupied by Lilian’s Lovebirds (binomial glm; log-odds ratio: 19.7 (7.8 − 29.6 CI), n = 54, P <0.001; Figure 2).
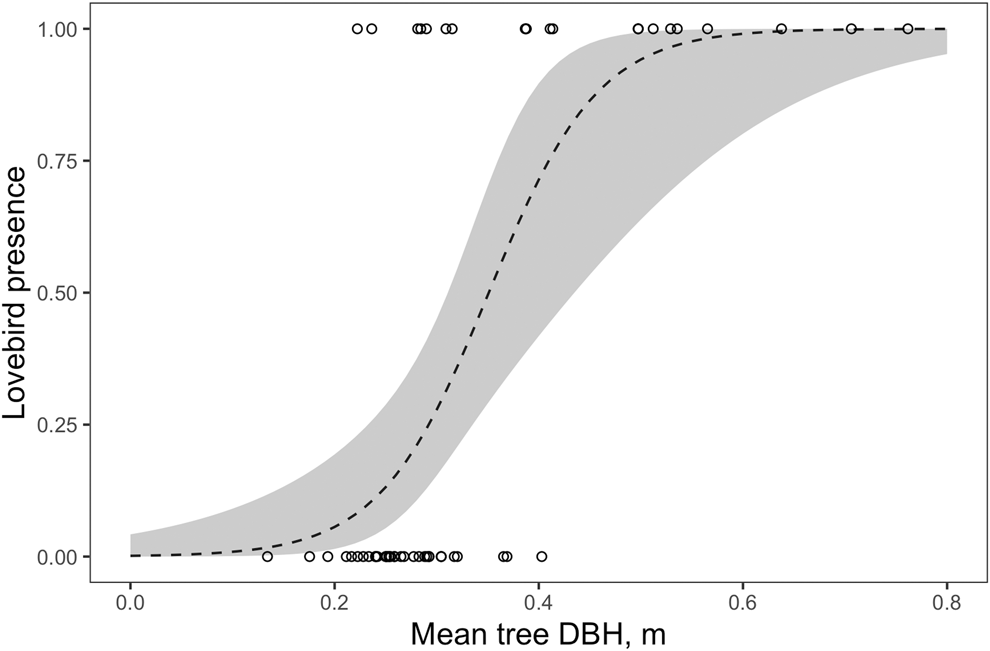
Figure 2. The presence of Lilian’s Lovebirds as a function of mean tree DBH. Line indicates prediction from binomial model and dashed lines 95% confidence intervals.
Discussion
We found that the presence of Lilian’s Lovebirds was strongly associated with the distribution of large mopane trees, supporting previous suggestions (e.g. Clancey Reference Clancey1971, Forshaw Reference Forshaw1989) that the species is a mopane woodland specialist. This association suggests that areas of ‘cathedral’ mopane may be a key resource for Lilian’s Lovebirds, providing a possible explanation for the species’ patchy distribution within mopane woodlands and highlighting the potential significance of protecting areas of ’cathedral’ mopane woodland for the conservation of this ‘Near Threatened’ species. By quantifying this association, we provide guidance for habitat management, highlight the potential role of Lilian’s Lovebirds as an indicator of the status of mopane woodland and provide a basis on which to develop species distribution models with which to assess the global conservation status of the species.
Drivers of the association between lovebirds and large mopane trees
One plausible explanation for the strong association between large trees and lovebird occurrence is that stands of large mopane trees contain a sufficient density of naturally occurring cavities to serve as roosting and breeding sites. Tree DBH is a common forestry measurement often used by forest managers as indicative of the age of a tree (Lieberman and Lieberman Reference Lieberman and Lieberman1987, Lewis et al. Reference Lewis, Amar, Taylor, Grice and Smith2009). Cavities tend to occur more frequently in older trees and therefore old growth trees can be an important resource to cavity-dwellers as a source of naturally formed cavities (Summers Reference Summers2007, Cockle et al. Reference Cockle, Martin and Wesołowski2011). In the Southern Hemisphere, there are generally relatively few cavity excavators (Cockle et al. Reference Cockle, Martin and Drever2010), thus trees that form cavities naturally are of great importance for cavity-dwelling species (Beaven and Tongayi Reference Beaven and Tongayi2013).
Alternative, non-mutually exclusive explanations for the association between Lilian’s Lovebird occurrence and large mopane trees exist. It is possible that areas with larger mopane trees may also provide a greater diversity or density of grasses that form part of their diet during the breeding season (Mzumara et al. Reference Mzumara, Perrin and Downs2018). It may also be that large trees occur in areas of greater availability of surface water (rivers or waterholes) which may also be an important resource. Future research should focus on identifying the mechanisms that drive this association.
Implications for the conservation of Lilian’s Lovebirds
The association between large mopane trees and the occurrence of Lilian’s Lovebirds suggests that habitat degradation may be an important threat to wild populations. Mopane woodlands are an important resource to the people that live in this landscape for both food, timber and charcoal (Ryan 2016, Woollen 2016). Furthermore, Zambia and Mozambique issue legal licences to harvest mopane trees outside protected areas (Kowero et al. Reference Kowero, Campbell and Sumaila2003, Monjane Reference Monjane2009) and the timber industry typically targets large trees. We observed this legal extraction outside South Luangwa National Park in Zambia in areas where Lilian’s Lovebirds were present (T. Mzumara and H. Tripathi pers. obs.). In Malawi, individuals who had a legal licence to harvest mopane in Mozambique were arrested for illegally harvesting over 2,000 mature trees in a Malawian National Park. This combination of legal and illegal extraction of mopane trees further threatens the lovebird habitat.
The absence of the Lilian’s Lovebird in areas where they historically occurred in the Zambezi valley is most likely due to this loss of habitat. We observed that grazing by livestock has changed the structure of mopane in the area, causing it to be stunted. In areas where the habitat may have been suitable such as the Mutulanganga IBA, mopane trees are being illegally cleared/cut down at an alarming rate (TG pers. obs.). The method used to fell trees in this area is a further cause for concern as trees are burned at the roots, which leaves no potential for future coppicing (Figure S3).
In addition to habitat loss, other threats have been identified elsewhere in their range. A recent study in southern Malawi highlighted the risk to Lilian’s Lovebirds posed by the use of pesticides to poison waterholes to catch bushmeat inside protected areas (Mzumara et al. Reference Mzumara, Perrin and Downs2016a). Although historically trapped in large numbers for the pet trade, there has been no international trade in wild specimens since 2002 (www.trade.cites.org: UNEP-WCMC, downloaded 2 June 2018) and there have been no recent reports of illegal trapping or seizures, suggesting that trapping is unlikely to be currently a major threat.
Recommendations for conservation
Mopane has a patchy distribution in southern Africa and occurs in a variety of forms ranging from stands of large ‘cathedral’ trees to stunted and ‘shrub’ mopane (Van Voorthuizen Reference Van Voorthuizen1976, Mapaure Reference Mapaure1994, Sebego Reference Sebego1999, Makhado 2014). Existing vegetation maps describing the distribution of mopane woodland do not differentiate between these markedly different forms of mopane woodland. The development of vegetation maps that distinguish between different forms of mopane would be an important foundation on which to identify potentially important areas of habitat for Lilian’s Lovebirds, based on the findings of our study. Spatial analyses using remotely sensed data are currently underway to help understand additional drivers of distributions such as presence of surface water and the species status in the rest of its range.
Our current findings suggest that conservation efforts should focus on protecting habitat in key areas for the species and address the processes leading to the loss of large trees throughout the species’ range. North and South Luangwa National Parks were identified as important strongholds for Lilian’s Lovebirds. In addition, the Lower Zambezi National Park is also important as it now appears to contain the only remnant population in the Zambezi Valley in Zambia. Given the apparent importance of these sites, lovebirds should be integrated into management plans for these protected areas. Site-specific initiatives to protect other sites outside of protected areas such as Mutulanganga, recognised as an Important Bird Areas (Leonard Reference Leonard2005, BirdLife International 2017) should also be considered.
The drivers of habitat loss must also be addressed by making efforts to strike a balance between human needs, species conservation and other ecosystem services. Policies on the logging of mopane trees need to be reviewed to ensure that the impacts to areas of conservation importance are minimised. An intergovernmental approach throughout the species’ range would help reduce the threats to areas of key habitat, particularly given the risks posed by illegal logging across national borders. Where areas of important habitat are being harvested for charcoal production, which can be an important source of fuel and cash income for rural communities, alternative energy sources and livelihoods need to be developed (Woollen et al. Reference Woollen, Ryan, Baumert, Vollmer, Grundy, Fisher and Lisboa2016).
Finally, the Black-cheeked Lovebird Agapornis nigrigensis, categorised on the IUCN Red List as ‘Vulnerable’, is closely related and behaviourally and ecologically similar to Lilian’s Lovebird (Eberhard Reference Eberhard1998, Warburton Reference Warburton2003). Field investigations conducted in the late 1990s, found little evidence of habitat destruction within their range and did not consider habitat loss to be a major threat at that time (Warburton Reference Warburton2003). However they were found to nest in aggregations and roost exclusively in mature mopane trees (Warburton Reference Warburton2003, Warburton and Perrin Reference Warburton and Perrin2005a,Reference Warburton and Perrinb). Given the apparent contraction of the range of Lilian’s Lovebirds within similar habitat in the region there is an urgent need for further investigations of the status of Black-cheeked Lovebirds. In the meantime, it would be prudent to prioritise the protection of cathedral mopane within their historical range.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000370
Acknowledgements
We would like to thank the following for their varied support and resources that made this work possible: World Parrot Trust, Pamela and Neville Isdell, The British Ecological Society, BirdWatch Zambia, International Foundation for Science, Wildlife and Environmental Society of Malawi, Zambia Wildlife Authority, Museums of Malawi, FitzPatrick Institute of African Ornithology, birdwatchers who submitted their records, Tracks for Africa, Lower Zambezi and Mwambashi lodges and many others.






