Article contents
Effect of the ligand in the crystal structure of zinc oxide: an x-ray powder diffraction, x-ray absorption near-edge structure, and an extended x-ray absorption fine structure study
Published online by Cambridge University Press: 20 April 2016
Abstract
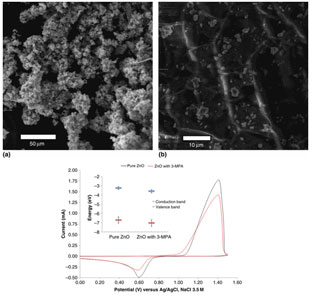
We analyze the effect of functionalization in the surface of zinc oxide crystal structure by 3-mercaptopropionic acid. X-ray powder diffraction data and extended x-ray absorption fine structure studies confirms a wurtzite structure. However, the morphology of the surface seems to be reduced and shows a film-like surface as demonstrated by x-ray absorption near edge structure and scanning electron microscopy. As a result of surface functionalization, the energy levels of the semiconductor were shifted toward reductive potentials (by 50 mV) as determined by diffuse reflectance and cyclic voltammetry.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
- 1
- Cited by





