Iodine is an essential mineral for thyroid hormone function( Reference Bath, Combet and Scully 1 , Reference Zou, Ding and Lou 2 ). Iodine-deficiency disorders (IDD) are still a public health problem in some countries( Reference Karwowska and Breda 3 ). The WHO estimates that nearly two billion people worldwide have insufficient iodine intakes, including one-third of all school-aged children( 4 , Reference Moleti, Sturniolo and Trimarchi 5 ).
Iodine is found in small amounts in many foods( Reference Slupczynska, Jamroz and Oda 6 ), but major sources are the marine foods group, milk and dairy products, and also drinking-water in some areas( Reference Watts, Joy and Young 7 ). Iodine deficiency is associated with several abnormalities which are named as IDD( Reference Roy, Chaturvedi and Agrawal 8 ). Some complications of iodine deficiency include goitre, lack of physical and mental development, and poor learning ability in children( Reference Osei, Baumgartner and Rothman 9 – Reference Chao, Zhang and Liu 11 ). Iodine deficiency also leads to abortion, stillbirth and congenital anomalies, and increases prenatal death and infant mortality( Reference Bath, Combet and Scully 1 , Reference Combet, Ma and Cousins 12 – Reference Chakraborty, Mazumdar and Chakraborty 14 ). IDD are preventable using methods such as iodized salt( Reference Zahidi, Zahidi and Taoufik 15 , Reference Gartner 16 ). The elimination of IDD should be implemented as a critical development issue by governments.
Recognizing the importance of IDD elimination, in 1994, the WHO and UNICEF Joint Committee on Health Policy recommended universal salt iodization (USI) as a safe, cost-effective and sustainable strategy to eliminate IDD( Reference Karwowska and Breda 3 , 4 , Reference Pandav 17 ). The USI strategy is recommended because salt is consumed by everyone and the quantity of iodine in salt can be monitored simply at the production, distribution and household levels( Reference Maalouf, Barron and Gunn 18 – Reference Lazarus 20 ). Most iodine absorbed in the body eventually appears in the urine. Therefore, median urinary iodine concentration (UIC) is the main indicator to be used to assess the iodine status of a population( Reference De Zoysa, Hettiarachchi and Liyanage 21 , Reference Nimmons, Funk and Graham 22 ).
Studies in the Islamic Republic of Iran showed that goitre was endemic in the majority of provinces and the total goitre rate (TGR) in the country was 68 %, being 30–80 % in school-aged children( Reference Emami, Shahbazi and Sabzevari 23 – Reference Azizi, Sheikholeslam and Hedayati 25 ). Therefore, the first law requiring mandatory iodization of all salt for household use was passed in 1994. Multiple rapid surveys on iodized salt consumption demonstrated that, from 1997, more than 90 % of households consumed iodized salt( Reference Azizi, Mehran and Sheikholeslam 26 ). Because more than 75 % of households in Azerbaijan Province consume marine foods including fish rarely or never, the main source of iodine is therefore iodized salt( Reference Saeidlou, Babaei and Ayremlou 27 ). Regular surveys of median UIC levels in school-aged children where feasible are useful indicators of thyroid function. School-aged children are a useful target group for IDD surveillance because of their combined high vulnerability, easy access and applicability to a variety of surveillance activities. The aim of the current study was to assess the TGR and UIC in school-aged children in the West Azerbaijan Province of north-west Iran between the years 2007 and 2015.
Methods and materials
In the present cross-sectional study, TGR, UIC, percentage of iodized salt consumption by households and iodine content of salt at production, distribution and household levels were assessed in schoolchildren in the north-west of Iran in 2007–2015. This study was approved by the ethics committee of Urmia University of Medical Science (Urmia, Islamic Republic of Iran).
Subjects
Two hundred and forty schoolchildren of both sexes, aged 8–10 years (grades 2, 3 and 4), were included. A multistage stratified sampling followed by a systematic random sampling technique was used to recruit participants (a separate sample was taken each year). According to the national guidelines, forty-eight schools with five samples from each school randomly should be selected. Initially, all primary schools were stratified into urban and rural. Therefore, considering the percentage of the rural and urban population (approximately 58 % in urban and 42 % in rural areas), twenty-eight schools in urban and twenty schools in rural areas (equal numbers of both sexes in each area) were selected by systematic random sampling. In this way, the first school was randomly selected; then, according to the sampling interval, the next schools were selected until the last school. The sampling interval was calculated as the total number of schools in each area (stratified by sex) divided by the number of schools needed. Finally, in each school, one subject for grade 2 and two subjects each for grades 3 and 4 were randomly enrolled.
Goitre grading and urinary iodine concentration measurement
Goitre grading was determined by physicians trained by one of the researchers in Tehran. Classification of the goitre grading was performed according to the criteria recommended by the WHO/UNICEF/International Council for Control of Iodine Deficiency Disorders (ICCIDD)( 4 ), as follows: grade 0, no palpable or visible goitre; grade 1, a goitre that is palpable, but not visible when the neck is in the normal position (i.e. the thyroid is not visibly enlarged; thyroid nodules in a thyroid which is otherwise not enlarged fall into this category); and grade 2: a swelling in the neck that is clearly visible when the neck is in a normal position and is consistent with an enlarged thyroid when the neck is palpated.
One hundred and twenty subjects were randomly selected for UIC determination (equal numbers of girls and boys). Urine samples were transferred on ice to the reference laboratory of Urmia University of Medical Sciences in screw-topped plastic bottles and kept frozen at –20°C until the time of iodine measurement at the end of the study. All laboratory measurements were done in one laboratory by physicians trained in using the acid digestion method. Based on the WHO/UNICEF/ICCIDD recommendation( 4 ), UIC was classified as follows: <20 μg/l as indicating severe iodine deficiency; 20–49 μg/l as moderate iodine deficiency; 50–99 μg/l as mild iodine deficiency; 100–199 μg/l as adequate iodine; 200–299 μg/l as above requirements; and ≥300 μg/l as excessive iodine.
Iodine content of salt
At household level
After an interview with parents about the type of household salt used, iodine content of the salt present in the household was measured in the field using a rapid testing kit. Four hundred samples were selected using simple one-stage cluster sampling. According to the national guidelines, twenty clusters with twenty samples in each cluster randomly should be recruited. Therefore, considering the percentage of the rural and urban population (approximately 58 % in urban and 42 % in rural areas), twelve clusters for the urban and eight clusters for the rural areas were chosen. Finally, households were randomly selected within the clusters. Approximately 10 % of salt samples were randomly selected and assessed using the iodometric titration method for quantifying iodine content, and values are shown in parts per million (ppm). Iodine level was recorded as <20, 20–40 and ≥40 ppm.
At production and distribution levels
Samples from different parts of the factory site at each factory producing iodized salt, and 100 samples from distribution sites with different brands, were sent to the laboratory of food and drug deputy in Urmia University of Medical Sciences. Quantitative iodine measurement was performed at the centre.
Statistical analysis
The data are presented as medians, means and standard deviations, or numbers and percentages. The normal distribution of data was tested using the Kolmogorov–Smirnov test. Mean UIC was compared by sex and area using the Mann–Whitney U test. Pearson’s χ 2 test was used for the comparison of iodine status distribution of subjects by sex and area. All statistical analyses were performed using the statistical software package IBM SPSS Statistics version 20. P values of less than 0·05 were assumed to be statistically significant.
Results
Urinary iodine concentration
Table 1 shows the urinary iodine levels in schoolchildren between 2007 and 2015. A total of 240 school-aged children were included in each year. Median UIC was ≥100 μg/l in all years except 2010 and 2011. There was no significant difference in mean UIC between boys and girls between 2007 and 2015. As the results show, the median and mean UIC were greater in urban children than in rural children in all years except 2012 and this difference was significant in all years except 2011 and 2012. Also, regular surveys from 2003 to 2006 indicated that the median UIC was ≥100 μg/l. Before the USI programme, it was <100 μg/l (data not shown).
Table 1 Urinary iodine concentration (UIC) by gender and area among schoolchildren aged 8–10 years (n 240 per year, selected by systematic random sampling each year) in West Azerbaijan Province, north-west Iran (2007–2015)

* P value from Mann–Whitney U test comparing between boys and girls.
† P value from Mann–Whitney U test comparing between urban and rural areas.
‡ Number of children for both urban and rural areas was 120 subjects in 2007.
Totally, the highest frequency of children with severe deficiency (UIC<20 μg/l) was 21·3, 13·3 and 8·8 % in 2012, 2011 and 2010, respectively, while its frequency was ≤5 % in other years. More than 50 % of children had insufficient iodine status (UIC≤99 μg/l) in both 2010 and 2011, while this rate was approximately 15–35 % in other years. More than 65 % of schoolchildren had UIC>100 μg/l in all years except 2010 and 2011. The highest and lowest rate of UIC≥300 μg/l was observed in 27·5 and 5·4 % of children, respectively. The proportion with UIC<50 μg/l was <20 % and with UIC>100 μg/l was more than 50 % among schoolchildren in all years except 2010 and 2011. Of children, 26·5 and 22·3 % had UIC<50 μg/l and 45·0 and 40·4 % had UIC>100 μg/l in 2010 and 2011, respectively. The lowest frequency of children with UIC<50 μg/l was 6·9 % in 2008 (Table 2).
Table 2 Iodine status distribution among schoolchildren aged 8–10 years (n 240 per year, selected by systematic random sampling each year) in West Azerbaijan Province, north-west Iran (2007–2015)

UIC, urinary iodine concentration.
Our findings showed that in six years the frequency of UIC≤99 μg/l was higher in boys than in girls; so that among boys its highest frequency was 55·0 % in 2010 and its lowest frequency was 16·7 % in 2013, while for girls these rates were 55·0 % in 2011 and 15·0 % in both 2008 and 2013, respectively. In all years, more than 50 % of both boys and girls had UIC>100 μg/l; except its frequency among both boys and girls was 45·0 % in 2010 and 2011, respectively. The highest frequency of excessive iodine status (UIC≥300 μg/l) was 31·7 % in 2015 and 27·5 % in 2008 for boys and girls, respectively. There was no significant difference in iodine deficiency by sex in all years except 2007 (P=0·02; Table 3).
Table 3 Comparison of iodine status distribution by sex among schoolchildren aged 8–10 years (n 240 per year, selected by systematic random sampling each year) in West Azerbaijan Province, north-west Iran (2007–2015)
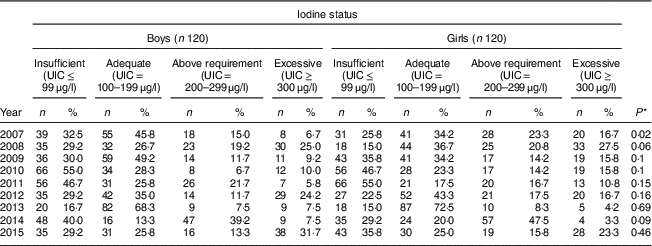
UIC, urinary iodine concentration.
* P value from χ 2 test comparing between boys and girls.
Table 4 shows the comparison of iodine status distribution of the schoolchildren by area. Rural children had iodine deficiency more than urban children in all years except 2012 and this difference was statistically significant in 2008, 2009, 2010 and 2015. The highest frequency of UIC≤99 μg/l was 62·0 % and 47·9 % in rural and urban children, respectively.
Table 4 Comparison of iodine status distribution by area among schoolchildren aged 8–10 years (n 240 per year, selected by systematic random sampling each year) in West Azerbaijan Province, north-west Iran (2007–2015)
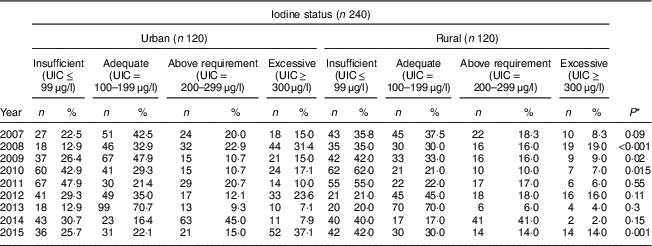
UIC, urinary iodine concentration.
* P value from χ 2 test comparing between boys and girls.
Elimination indicators of iodine-deficiency disorders
Elimination indicators of IDD are shown in Table 5. Iodized salt was consumed by 98 % of households. The TGR was 0·4 % and 97 % of salt samples examined at the household level by rapid testing kit showed iodization. Iodine levels <20, 20–40 and >40 ppm were observed in 12·5, 69·5 and 18·0 % of salt at the production level; 17·0, 52·0 and 31·0 % of salt at the distribution level; and 27·0, 52·5 and 20·5 % of salt at the household level, respectively, in the last national survey (in 2007). According to national guidelines, goitre was evaluated every 5 years in children. Because of the reduction of TGR to less than 5 % according to WHO/UNICEF/ICCIDD criteria( 4 ), the last survey was conducted in 2007. The first national survey 2 years after iodization of salt in 1996 showed that the TGR was 44 % among children aged 8–10 years in West Azerbaijan Province. TGR decreased to 7·6 and 0·4 % in 2001 and 2007, respectively (Fig. 1). Regular surveys from 2002 to 2015 showed that 98 % or more of households consumed iodized salt. The last national study in 2014 showed that the median iodine content of household salt in West Azerbaijan was 27 ppm (data not shown).

Fig. 1 Total goitre rate (TGR) among schoolchildren aged 8–10 years in three national surveys in West Azerbaijan Province, north-west Iran
Table 5 Sustainability indicators of elimination of iodine-deficiency disorders in the West Azerbaijan Province, north-west IranFootnote *
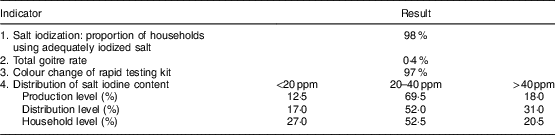
* Data from the last national survey in 2007.
Discussion
According to WHO statistics, IDD remain a public health problem in several countries( 4 ). There are still numerous places in the world where iodized salt is not available( Reference Karwowska and Breda 3 ). Regarding the adverse effects of iodine deficiency on community health( Reference Osei, Baumgartner and Rothman 9 , Reference Abebe, Gebeye and Tariku 28 ), baseline IDD prevalence studies and regular follow-up surveys, including measurement of urinary iodine levels and an analysis of the salt situation, are necessary. The current study aimed to assess the UIC, TGR and the sustainability of elimination indicators of IDD in the West Azerbaijan Province of north-west Iran in nine consecutive years.
The current study showed that mean and median UIC were not significantly different between boys and girls. In six years, iodine deficiency (UIC≤99 μg/l) was more prevalent in boys than girls. Fifty-five per cent of children had iodine deficiency (UIC≤99 μg/l) in both 2010 and 2011, while this rate was approximately 15–35 % in other years. McDonnell et al. reported that the frequency of iodine deficiency was 68 % in boys and 79 % in girls in Melbourne, Australia( Reference McDonnell, Harris and Zacharin 29 ). According to the WHO/UNICEF/ICCIDD recommended criteria( 4 ), the indicator of IDD elimination is a median value for UIC of 100 μg/l, and UIC should not be lower than 50 μg/l in more than 20 % of subjects. Also, based on these criteria, more than 50 % of children have should have UIC above 100 μg/l( 4 ). In the current study, 26·5 and 22·3 % of children had iodine level below 50 μg/l only in 2010 and 2011, respectively, while this rate was less than 20 % in other years, so that its lowest frequency was 6·9 % in 2008. More than 50 % of schoolchildren had UIC>100 μg/l in all years except 2010 (45·0 % of children) and 2011 (40·4 % of children). Median UIC less than 100 μg/l in these two years is due to rural areas. Its possible cause may be the rural areas that are near to Lake Urmia, because these rural areas use lake salt instead of iodized salt. In two studies by Keshteli et al. and Khalili et al. in the central area of Iran, 16 % of children had UIC below 100 μg/l and 3·7 % had UIC below 50 μg/l( Reference Keshteli, Hashemipour and Siavash 30 , Reference Khalili, Hashemipour and Keshteli 31 ). Zimmermann reported that the prevalence of UIC<100 μg/l in schoolchildren was 40 %, and children with UIC>100 μg/l increased from 60 to 86 % in 1999 and 2004, respectively( Reference Zimmermann 32 ).
Our findings showed that rural children had iodine deficiency more than urban children and this difference was significant in some years. The results of Chirawurah and Addah’s study showed that iodized salt consumption in rural households of northern Ghana was low; 20 % of households used iodized salt for cooking while 80 % did not cook with iodized salt( Reference Chirawurah, Apanga and Addah 33 ). Gupta et al. indicated that the prevalence of goitre was 18 % in rural areas and 7·5 % in urban areas of Lucknow, India( Reference Gupta, Srivastava and Zaidi 34 ).
Results of the current study showed that 98 % of households consumed iodized salt and 97 % of salt samples examined at the consumer level by rapid test kit showed iodization. Iodine level ≥20 ppm was observed in 87·5, 83·0 and 73·0 % of salt at the production, distribution and household level, respectively. The TGR decreased from 44 % in 1996 to 7·6 % in 2001 and 0·4 % in 2007. According to the WHO/UNICEF/ICCIDD recommended criteria, 95 % of salt for human consumption must be iodized and a salt iodine content of 20–40 ppm by titration must be found in ≥90 % of a representative sample of households. Also, these criteria suggest that the TGR should be less than 5 % in schoolchildren( 4 ).
The availability and consumption of iodized salt in households in other studies have been reported as more than 90 %, consistent with the present results( Reference Zou, Wu and Guo 35 – Reference Doggui, El Ati-Hellal and Traissac 37 ). In a study by Kapil et al. conducted in Uttarakhand, India, only 46·7 % of the salt samples had iodine level ≥15 ppm( Reference Kapil, Pandey and Jain 38 ). In Jaiswal et al.’s study, the median (range) iodine concentration of household powdered and crystal salt was 55·9 (17·2–65·9) ppm and 18·9 (2·2–68·2) ppm, respectively, in Bangalore, India( Reference Jaiswal, Melse-Boonstra and Sharma 39 ).
Before the USI programme, goitre was endemic in the majority of provinces in Iran including West Azerbaijan( Reference Azizi, Navai and Fattahi 24 , Reference Azizi, Sheikholeslam and Hedayati 25 ). In a study by Azizi et al. in all provinces of Iran, the prevalence of goitre (grades 1 and 2) was 44 % in West Azerbaijan in 1996 (2 years after introduction of the law for the mandatory production of iodized salt)( Reference Azizi, Sheikholeslam and Hedayati 25 ). The results of another study by the same author indicated that the TGR decreased significantly from 53·8 % (in 1996) to 13·9 % (in 2001) after 7 years of uniformly iodized salt consumption by Iranian households( Reference Azizi, Mehran and Sheikholeslam 26 ). Khalili et al. reported the overall goitre rate was 32·9 % in schoolchildren of Isfahan in 2005( Reference Khalili, Hashemipour and Keshteli 31 ). The overall goitre rate was 36·7 % in another study in the same area( Reference Hashemipour, Siavash and Amini 40 ), while the overall goitre rate was 7·84 % in the study of Monajemzadeh and Moghadam in south-east Iran( Reference Monajemzadeh and Moghadam 41 ). The overall prevalence of goitre was 12·6 % among children aged 6–12 years of Ambala, Haryana, India( Reference Chaudhary, Pathak and Ahluwalia 42 ). Coccaro et al. reported that the goitre prevalence was 3·8 % among schoolchildren living in urban central Italy( Reference Coccaro, Tuccilli and Prinzi 43 ). The current study showed that the TGR was <5 %, which meets the WHO/UNICEF/ICCIDD criteria( 4 ).
In summary, after the USI programme, West Azerbaijan Province achieved the WHO criteria on IDD elimination. Our study showed that, based WHO/UNICEF/ICCIDD recommendations( 4 ), in the last national survey (13 years after USI) the TGR decreased to normal. The availability and consumption of iodized salt in households were 98 % or greater. The median UIC was ≥130 μg/l in all years except 2010 and 2011.
Conclusion
Our focused data suggest that the USI programme is improving the health of schoolchildren in the West Azerbaijan Province of north-west Iran since 1996, meeting all WHO/UNICEF/ICCIDD criteria for the sustainable elimination of IDD. The reduction of the TGR in schoolchildren to less than 5 % indicates the successful elimination of IDD as a major public health problem.
Acknowledgements
Acknowledgements: The authors would like to thank all respected experts in the health departments of Urmia University of Medical Sciences and other professionals who have cooperated in conducting this study. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declared no conflicts of interest. Authorship: F.B. collected the data. S.N.S. and R.E. contributed in designing and implementing the study and writing the paper. P.A. analysed the data and wrote the paper. Ethics of human subject participation: This study was approved by the ethics committee of Urmia University of Medical Science (Urmia, Islamic Republic of Iran).











