INTRODUCTION
Hantavirus pulmonary syndrome (HPS) is the most frequently reported fatal rodent-borne disease in Brazil. Since 1993, when the first HPS cases were identified in Brazil, over 1800 cases have been reported, accounting for more than 45% of HPS cases reported in the Americas. HPS cases have been confirmed in 15 federated units, most of them in the southern, southeastern and midwestern regions of the country. The global case-fatality rate is ~40% [Reference Iversson1–3]. Of the Brazilian states, the southern state Santa Catarina (SC) has the largest proportion of cases (15·5%, 291/1876). Curiously, the mortality rate in this state is ~29%, less than that found in the rest of the country [3]. This difference in mortality rates is most likely due to variations in the virulence or circulation of hantavirus genotypes as well as differences in the genetic characteristics and immune status of the patients and the epidemiological surveillance systems and adequacy of supportive medical care available in different areas.
With its expansive territory (8 514 215 km2), Brazil occupies most of the eastern portion of South America. This large country supports several biomes with multiple natural ecosystems that account for the reported regional differences and temporal trends of HPS [Reference Pinto Junior4, Reference Willemann and Oliveira5], highlighting the need for region-specific studies. To date, six hantavirus genotypes have been identified as being associated with HPS in Brazil: Juquitiba (JUQV), Araraquara, Castelo dos Sonhos, Laguna Negra, Anajatuba and Rio Mamore. These viruses are transmitted by wild rodent reservoirs of the species Oligoryzomys nigripes, Necromys lasiurus, O. utiaritensis, Callomys callidus, O. mattogrossae and O. microtis, respectively [Reference Johnson6–Reference Weksler, Bonvicino, Patton, Pardiñas and D'Elía13]. Two other hantaviruses, not yet associated with human disease, have also been found in wild rodents in Brazil: Jabora (JABV), obtained from Akodon montensis in the southern region, and Rio Mearim, obtained from Holochilus sciureus in the northern region [Reference Rosa8, Reference Oliveira14].
HPS is a nationally reportable disease in Brazil, and its surveillance is based on serological methods, most commonly the detection of virus-specific immunoglobulin M (IgM) in serum using an enzyme immunoassay (EIA). The classification of ‘confirmed’ HPS requires signs and symptoms consistent with having the disease and at least one confirmatory laboratory finding or an epidemiological link to a confirmed HPS case. Rarely is an alternative diagnostic method, such as reverse transcription–polymerase chain reaction (RT–PCR), available for surveillance, and consequently, the molecular identification of the hantavirus responsible for a case is not routinely performed in Brazil [15].
This retrospective study reviews the clinical, laboratory, and epidemiological findings in HPS cases diagnosed in the state of SC from 1999 to 2011 and reports features and a molecular analysis of six HPS cases from the midwest region of this state. We also summarize recent findings of hantavirus rodent reservoir species identified in the municipality of Jaborá in our previous study [Reference Oliveira16], one of the areas where human illness has occurred and where we have demonstrated the co-circulation of two different hantavirus genotypes, JABV (not related to human disease) and JUQV. Based on these data, it was possible to associate the HPS cases over a 3-year period with the virus genotype circulating in rodent reservoirs and the occurrence of rodent outbreaks.
MATERIALS AND METHODS
Study site
SC, located in the southern region of Brazil, is 95 736 km2 in size and has an estimated population of 6 727 148 people, with a population density of 65·27 inhabitants/km2. The climate is humid subtropical. SC is divided into six mesoregions: (i) western SC, (ii) southern SC, (iii) northern SC, (iv) mountain, (v) the Vale do Itajaí and (vi) the Grande Florianópolis (Fig. 1). Geographically, SC is divided into a narrow coastal plain on the east and a large plateau on the west. The original vegetation of the state was comprised of both forests and fields. Forests, occupying 65% of this state, have been greatly reduced due to devastating deforestation. In the plateau, mixed forests of conifers (pines) and hardwoods exist, whereas in the lowland and the slopes of the Serra do Mar, only broad-leaved forest exists. Fields occur as scattered patches within the mixed forest.
Fig. 1. Map of the state of Santa Catarina in southern Brazil, indicating the mesoregions (colours) and the municipalities where the six hantavirus pulmonary syndrome patients included in the molecular analysis lived (grey).
The economy is based on industry (especially agribusiness, textiles, ceramics and metal-mechanical), extraction (mining) and livestock. The main agricultural product of SC is corn, grown in the basalt plateau which provides feed for pigs.
Data analysis
In this retrospective study, we reviewed all of the confirmed cases of HPS in SC reported to the national HPS surveillance system between 1999 and 2011. The anonymized secondary data including the demographic, epidemiological, clinical, laboratory and patient outcome data collected by the Health Bureau of the State of Santa Catarina (HSSSC) were analysed.
Statistical analyses
Descriptive statistics were used, and the data are expressed as absolute frequencies for the qualitative variables and as the mean ± standard deviation for the quantitative variables. The χ 2 test was employed to establish possible associations between death caused by HPS and epidemiological, clinical, laboratory and radiological variables, considering significance levels of P < 0·05 and 95% confidence intervals (CIs). The analyses were performed using Microsoft Excel software v. 15.0 (Microsoft Corp., USA).
The odds ratio (OR) with 95% CI was used as a measure of association. For comparisons of more than two proportions, a logistic regression was performed to calculate the OR, using Epi Info v. 3.5.4 (CDC, USA).
In addition, a generalized linear model (GLM) analysis was performed in order to investigate the influence of independent variables on the response variable (evolution = recovery or death). As variables that are non-significant in the univariate analysis can become significant once adjusted by other variables in a multivariable analysis, those that presented an association in the univariate analysis with P⩽0·2 were included in the first model. Following this, a forward–backward stepwise procedure was used and the variables in the first model with a P value < 0·1 were retained in the final model. The OR of each variable was calculated and the 95% CI was used to confirm the effect of these independent variables on the response variable. All analyses were performed in R statistical software v. 2.13.1 (R Foundation for Statistical Computing, Austria).
The box-plot graphic was used to manually construct the class intervals when designing the map of HPS case numbers. This application of the box-plot function deserves attention because it aggregates information relevant to the map.
HPS patients and molecular sampling
Blood samples were collected from six patients in the western region of SC with HPS previously confirmed by a serological test (Fig. 1), and the extracted RNA was subjected to RT–PCR. Primers specific to the hantavirus S segment sequence were used to amplify and sequence the partial and complete genomic segment as reported previously [Reference Oliveira17, Reference Guterres18].
The partial S segment sequences obtained were aligned with Hantavirus sequences retrieved from GenBank. Multiple sequence alignment was performed using muscle in SeaView v. 4 software [Reference Gouy, Guindon and Gascuel19]. The phylogenetic relationships were estimated by a Bayesian Markov Chain Monte Carlo (MCMC) method implemented in MrBayes v. 3.1.2 [Reference Ronquist and Huelsenbeck20]. For the analyses, the sequence of Seoul virus (Genbank no. AY 627 049) was used as the outgroup species.
Additional sequences retrieved from four O. nigripes specimens trapped in the Jaborá municipality, Oln8057 (Genbank accession no. JX 173 803), Oln8091 (Genbank accession no. JX 173 804), Oln9592 (Genbank accession no. JX 173 805), and Oln9845 (Genbank accession no. JX 173 806), were included in the analysis. These samples were obtained during ten small mammal capture expeditions that were conducted between 2004 and 2006 in the municipality of Jaborá in our previous study [Reference Oliveira16].
Ethics statement
The Ethics Review Board of the Oswaldo Cruz Foundation (FIOCRUZ) approved the project under no. 559/10. Informed consent was obtained from HPS patients from the midwest region of SC whose blood was used in the molecular sampling study.
RESULTS
Epidemiological, clinical and laboratory findings
During the study period, November 1999 to December 2011, 251 HPS cases were reported to the HSSSC. The majority (93·2%) of these cases were confirmed by serological assay, but 17 (6·8%) cases were only confirmed using clinical-epidemiological criteria. Of the cases confirmed by serology, nine cases were also confirmed by RT–PCR, and nine processed samples were confirmed by immunohistochemistry. Only one case was confirmed by three specific tests.
The distribution of cases in SC during the study period is shown in Figure 2. The highest annual number of HPS cases was observed in 2006 (n = 47), followed by 2004 (n = 44). The global case-fatality rate during the period from 1999 to 2011 was 29·1%, with annual rates ranging from zero (1999 and 2000) to 58·3% (2009). The morbidity and fatality rates were 0·3 and 0·9 cases/million persons, respectively. Cases occurred throughout the year, although November and December were the peak months of illness onset (data not shown). During the study period, the western SC mesoregion accounted for 49·8% of HPS cases but had the lowest case-fatality rate (19·2%). Cases occurred in all six mesoregions, including a total of 113 municipalities, but only four (1·59%) cases have been confirmed in the southern SC mesoregion since 2008 (Fig. 3). In the second most frequently affected region, the Vale do Itajaí (19·5% of cases), the case-fatality rate was high (48·9%). The case-fatality rates in the different geographical mesoregions varied significantly and the highest proportions of fatal cases were seen in patients from north SC (P = 0·01) and the Vale do Itajai (P < 0·01) regions, where the analysis of ORs for death by HPS were 4·13 and 3·96, respectively (Table 1). The morbidity rate ranged from 0·03 cases/million persons in the south to 0·83 cases/million persons in the west.
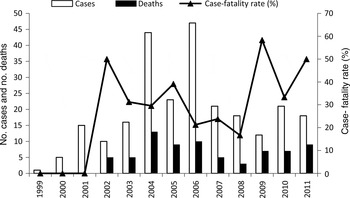
Fig. 2. Number of annual hantavirus pulmonary syndrome cases, related deaths and case-fatality rates in the state of Santa Catarina, Brazil (1999–2011).

Fig. 3. Hantavirus pulmonary syndrome case distributions in Santa Catarina (SC) and its mesoregions in 1999–2011. The number of cases is represented by class intervals indicated by the colors. Rodent outbreaks or ratadas reported in some municipalities of SC during 2004–2006 are represented by rat images on the map.
Table 1. Epidemiological characteristics of hantavirus pulmonary syndrome (HPS) cases in the state of Santa Catarina, Brazil (1999–2011)

OR, Odds ratio; CI, confidence interval.
* Data not available in all HPS cases.
† Case-fatality rate for all case-patients.
‡ P value for testing the difference in proportion of HPS case-patients who died between categories for each characteristic.
In general, since 1999, most of the HPS cases in SC have occurred in the western and midwestern regions of the state, although a number of cases were reported in the eastern region in 2002, and in 2004 other mesoregions were affected. From 2004 to 2006, 18 municipalities reported rodent outbreaks after bamboo mast events; 15 of these events were associated with confirmed HPS cases (Fig. 3).
The data analysis of HPS cases collected by HSSSC showed that the mean HPS patient age was 34·560 ± 13·381 years (range 0–73 years). Most patients (74/250, 29·6%) were aged between 40 and 49 years, and 61/250 (24·4%) were aged 30–39 years. No deaths were observed in children aged <10 years, an age group that accounts for 2·8% of the cases. No significant difference in case-fatality rates were noted in the age groups (Table 1).
The HPS population had a higher proportion of males than females (80·8% vs. 19·1%); the male/female ratio was 203/48 (4·2/1) (Table 1). The case-fatality rate between genders was not significantly different: 33·3% females vs. 28·1% males (P = 0·47) (Table 1).
The probable site of infection was related to the rural environment in 164/234 cases (70·1%), and 150/234 HPS patients (64·1%) reported contact with rodents or rodent excreta (Table 1). An occupational exposure that includes agricultural, livestock and forest activities was reported by 108/180 patients (60%) for whom such information was available.
The frequencies of selected clinical and laboratory findings available from the health service archive are reported in Table 2. We compared them with the main published series in Brazil (Table 3). Fever was the most consistent symptom, present in 95% of HPS cases (233/246). The other most common non-specific clinical findings were headache (88·3%), myalgia (74·1%), vomiting (77%) and abdominal pain (63·6%). More specific and frequent clinical findings were thoracic pain (53·4%), dyspnoea (72·1%), cough (61·3%) and acute respiratory failure (43·8%). Acute renal failure was observed in 33·0% of HPS patients, and petechiae and/or bleeding were observed in 15% of the patients. Neurological manifestations were apparent in 11% of the patients.
Table 2. Clinical and laboratory characteristics of hantavirus pulmonary syndrome (HPS) cases in the state of Santa Catarina, Brazil (1999–2011)

OR, Odds ratio; CI, confidence interval; BUN, blood urea nitrogen.
* Data not available in all HPS cases.
† Case-fatality rate for all case-patients.
‡ P values were determined by χ 2 test, excepted for fever data, which was evaluated by Fisher's exact test, for testing the difference in proportion of HPS case-patients who died between categories for each characteristic.
Table 3. Clinical and laboratory findings in patients diagnosed with hantavirus pulmonary syndrome in Brazil in the present study and in other published series

HSS, Health Surveillance Secretariat; NI, not informed; BUN, blood urea nitrogen.
* Increase of urea.
† Increase of creatinine.
Blood tests revealed thrombocytopenia in 73%, leukocytosis in 45·1% and atypical lymphocytes in 34·7% of HPS patients. Chest radiographs, when available, showed diffuse pulmonary infiltrates in 121/192 cases (62·3%).
Dyspnoea, acute respiratory failure, renal failure, increased haematocrits, increased serum creatinine and blood urea nitrogen (BUN) levels, and the presence of pulmonary interstitial infiltrate were significantly more common in HPS patients who died than in HPS patients who survived (P < 0·001) (Table 2). According to univariate analyses these clinical and laboratory variables mentioned above, and also fever, thoracic pain, thrombocytopenia and the presence of atypical lymphocytes (P < 0·2), age group (20–29 years, P = 0·07) and localities (mesoregions, P ⩽ 0·01) were considered for the first model in GLM. The only retained variables in the model were localities (mesoregions) and increase of BUN and creatinine levels, although only the later was significant by OR and 95% CI (Table 4), indicating that this variable were statistically associated with evolution to death.
Table 4. Risk factors for death by hantavirus pulmonary syndrome according to final model in GLM, Santa Catarina, Brazil (1999–2011)

OR, Odds ratio; CI, confidence interval; BUN, blood urea nitrogen.
* Significant by odds ratio.
Patients were hospitalized in 236/245 cases (96·3%), and the fatality rate was 30·1%. The mean time from onset of symptoms to death was 3·6 ± 3·8 days. The patients were treated with mechanical ventilation in 94/227 cases (41·4%) for which data were available. Antiviral treatment with ribavirin was used in only 21 patients, and 15 (71·4%) of these patients recovered. No information on the use of vasoactive agents was available in the database.
Molecular data
For the six HPS cases in which a molecular analysis was performed (Supplementary Table S1), the nucleotide sequences of the S segment of viral RNA confirmed a JUQV infection. Five patients were included in the phylogenetic analysis and grouped with a well-supported clade composed of JUQ-like viruses, as shown in the phylogenetic tree (Supplementary Fig. S1). A complete sequence of the JUQV S segment (~1900 bp) was obtained from one of the human HPS samples (case 4, LH0076/11) and was also included in the phylogenetic analysis (Genbank accession no. JX 173 798).
Figure 4 shows the number of HPS cases occurring between 2004 and 2006 in the municipality of Jaborá in association with the number of infected wild rodents captured during the same period [Reference Oliveira16]. In this hantavirus-reservoir study, both JABV and JUQV genotypes were identified in 8/10 trapping expeditions; in December 2005 and 2006, only JUQV was found. The highest incidence of HPS cases was found in November and December and was associated with the highest number of animals infected with JUQV.

Fig. 4. Temporal correspondence of hantavirus pulmonary syndrome (HPS) cases and reservoir infection over a 3-year period. Blue bars, number of HPS cases in humans per month for the state of Santa Catarina (Brazil) from 2004 to 2006; black dots, observed hantavirus antibody prevalence in A. montensis (JABV host) by trapping session; red dots, observed hantavirus antibody prevalence in O. nigripes (JUQV host) by trapping session in Jaborá over the same period. Right scale, percentage of seropositive rodents in the trapped sample.
DISCUSSION
The present study assessed the clinical, epidemiological and laboratory characteristics of patients with HPS and identified the hantavirus genotype responsible for causing human infection in the midwestern portion of SC, Brazil. The frequency of the clinical and laboratory findings was in accordance with other studies conducted in Brazil, with fever, myalgia and headache being the most frequently reported clinical manifestations [Reference Raboni21–Reference Elkhoury24]. Gastrointestinal tract manifestations such as vomiting and abdominal pain, which can confound the diagnosis and lead to inappropriate therapy, were found in 77% and 63·3% of the patients, respectively. Although renal failure is not a common symptom of HPS, it was associated with a case-fatality rate of 52·6%; most of the fatalities (80%) occurred within 5 days of onset of symptoms. According to the multivariable analysis, high serum urea nitrogen and creatinine levels are significantly associated with death, emphasizing the importance of biochemical analysis. As noted for other Brazilian regions, most cases of HPS are associated with the rural environment, where biochemical specific assays are usually not performed, only X-ray and complete blood count (CBC) are commonly performed. In this scenario, HPS can resemble many other infectious diseases, especially dengue and leptospirosis, two of the most frequent endemic infectious diseases in Brazil. Such a misdiagnosis can lead to vigorous fluid administration, which may aggravate the respiratory failure associated with pulmonary inflammation in HPS. The frequency of mechanical ventilation, 41·4%, was similar to the 46·5% reported by a large study of 855 HPS patients in Brazil [Reference Elkhoury24].
Although early healthcare management would be expected to be associated with a lower fatality rate, the highest fatality rate occurred in patients admitted to the hospital within the first 5 days of onset of symptoms, suggesting the occurrence of fulminant cases of HPS. There was no difference in fatality between male and female patients, a finding that differs from that of a previous study [Reference Martinez25]. HPS presents with a broad clinical spectrum, ranging from asymptomatic to oligosymptomatic, to fatal with fulminant pulmonary oedema. Host-related factors, the viral genotype and virus load might explain this wide variation [Reference Khan26–Reference MacNeil, Ksiazek and Rollin28]. Some hantaviruses are considered non-pathogenic to humans, although other factors that appear to contribute to the severity of the clinical characteristics can be related to the human host (histocompatibility complex) [Reference Plyusnin29–Reference Kilpatrick31].
Although not confirmed by the OR analysis, the greater probability of the occurrence of death by HPS in SC was also associated with the locality; the highest fatality rate was observed in the northern SC and Vale do Itajaí regions. This result could be associated with the greater attention from health services in some regions, especially during the years with the largest number of cases. This could explain why although western SC has the highest number of cases it still shows the lowest fatality rate. Our result reinforces the need for adjusting surveillance tools and investigating other factors associated with this different fatality rate in SC.
In our series, the epidemiological results were consistent with the data reported in the literature [Reference Raboni21–Reference Elkhoury24]. Our results were consistent with the characterization of HPS as a disease of rural, predominantly male, agricultural workers in their productive years. The majority of cases occurring in residents of rural areas and agricultural and livestock (farming) activities are correlated with the risk of exposure to hantavirus in 60% of cases, characterizing HPS as a clearly work-related disease. Contact with rodents is extremely common in rural locations, and as expected, 64·1% of the SC patients reported contact with rodents or rodent excreta.
Only seven (2·8%) cases occurred in children aged <10 years. Reports of HPS in individuals aged <17 years are uncommon. In the United States, <1% of HPS cases were reported in individuals aged <10 years, and <7% of HPS cases occurred in children aged <17 years [Reference MacNeil, Ksiazek and Rollin28, Reference Ramos32, 33]. In Argentina, 9·3% of HPS cases were in children aged <14 years [Reference Martinez25]. All of the clinical illnesses of the paediatric patients in these reports were similar to those observed in adult patients in these studies, as was the case in our study.
Unlike that observed in the country as a whole or in other regions, HPS has been reported to display a clear seasonal trend in the southern region of Brazil [Reference Pinto Junior4]. Similarly, in SC, the fact that the greatest number of cases was recorded between October and December suggests seasonality, probably related to the seasonal harvesting and storing of seeds (grains), especially corn. Thus far, the emergence of isolated cases or outbreaks of HPS in Brazil has been connected to particular situations or environmental risk factors, such as (1) an agricultural profile, as observed in most cases in SC, with the involvement of cornfields bordering forests; (2) an association with the construction of bunkers or other buildings that allow the entry of rodents and their consequent direct access to stored food or seeds; or (3) occupational exposures in which an employee might encounter a rodent or rodent droppings (e.g. corn bags) in cornfields or other crop seed fields. In general, the greatest risk of exposure to hantavirus is associated with entering closed buildings that are rodent-infested [Reference Raboni21–Reference Elkhoury24, Reference Pinto, de Sousa and de Lemos34]. Other factors, such as climate change, characteristics of the biotic environment (e.g. habitat quality and agricultural fields) and human activities and behaviour must be included as risk factors for sporadic outbreaks of hantavirus.
In SC, during the outbreak years of high incidence (2004 and 2006), HPS cases were detected mainly in municipalities where rodents outbreaks were reported after a widespread bamboo mast event. The ‘taquara-lixa’ bamboo is endemic to the south Atlantic Forest, and had mast flowered in some areas of SC during 2004, 2005 and 2006. Known as ratada, the phenomenon of rodent outbreaks has been recorded in South America since the Spanish conquest, more specifically, in Brazil and Chile [Reference Pereira35–37]. In 2006, 50% of the HPS cases were reported in municipalities in which mast events occurred, as observed in the mesoregions of Vale do Itajai and Grande Florianópolis. This fact attracted the attention of the staff of the State Epidemiological Surveillance at the time (Epidemiologic Surveillance of Santa Catarina State, Report on Hantavirus cases 1999–2011, unpublished data). Thus, the information obtained in this historical series coupled with the occurrence of ratada reinforces a possible direct association between bamboo mast events, rodent population outbreaks and HPS cases.
In a previous longitudinal study of hantavirus infection in rodents in Jaborá, located in the midwestern region of the state, the co-circulation of two different genotypes in three different rodent species was reported: JABV in Akodon paranaensis and A. montensis and JUQV in O. nigripes [Reference Oliveira14, Reference Oliveira38]. JABV in A. montensis and JUQV in O. nigripes were also observed by Chu et al. in Paraguay [Reference Chu39]. Our previous study described the genetic analysis performed on samples from rodents captured at the presumed site of infection of one human patient (case 4, Supplementary Table S1) during the mast seeding events in Jaborá [Reference Oliveira14]. Furthermore, we found that highest number of infected animals with JUQV was found in November and December, months with a high incidence of human HPS cases in SC.
Greater awareness of the fact that HPS can occur simultaneously with dengue or leptospirosis is needed, especially in critically ill patients, in whom the diagnosis can easily be missed if not suspected, and vigorous fluid administration may aggravate the respiratory failure associated with pulmonary inflammation. Emergency physicians should be aware of rodent outbreaks and be vigilant for hantavirus exposures, including ecotourism activities, especially during the summer and early autumn. The observation that ratadas occurred during the years with a high incidence of HPS cases in SC underscores the importance of analysing the association of HPS with bamboo mast events and rodent population outbreaks, especially in southern Brazil. Moreover, the possible existence of another hantavirus genotype associated with asymptomatic infection or mild disease could explain the lower case-fatality rate in this area of Brazil and should be continuously investigated.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268815002460.
ACKNOWLEDGEMENTS
We thank Luciana Helena Bassan and Endiá Almeida for technical support (IOC/FIOCRUZ). We also thank Maria Angélica Mares-Guia, Mônica de Avelar Figueiredo Mafra Magalhães e Renata de Saldanha da Gama Gracie Carrijo (Sector for geoprocessing/ICICT) for image production and treatment assistance, FIOCRUZ. We are also grateful to Bernardo Rodrigues Teixeira for assistance in the revision and final analysis.
DECLARATION OF INTEREST
None.













