No CrossRef data available.
Article contents
Segregation of Yttrium Ions as 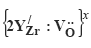 to the Surfaces of t-ZrO2
to the Surfaces of t-ZrO2
Published online by Cambridge University Press: 11 February 2011
Abstract
Atomistic simulation has been used to predict the segregation of defect clusters containing two substitutional Y3+ ions and one charge compensating oxygen vacancy to the (100) and (101) surfaces of t-ZrO2. The most stable orientation of the defect cluster depends on its distance from the surface. Significantly, segregation energies vary greatly between surfaces. For example, the defect cluster is equally stable up to a depth of 9Å from the (100) surface but only to a depth of 4Å below the (101) surface. In both cases, segregation energies are negligible 12Å beneath the surface.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 2003




