Article contents
Low-temperature processing and control of structure and properties of TiO2/c-sapphire epitaxial heterostructures
Published online by Cambridge University Press: 24 April 2013
Abstract
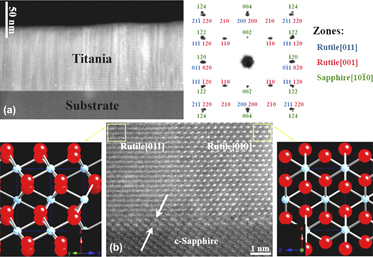
We have investigated the formation of the rutile and the anatase polymorphs of TiO2, with emphasis on epitaxial growth characteristics, and defect content as a function of laser and substrate variables. X-ray diffraction (XRD) studies revealed that the rutile phase is more stable at higher substrate temperatures and lower oxygen pressures; in contrast, decreasing the temperature and increasing the oxygen pressure gave rise to formation of anatase. Epitaxial rutile films with a <100] orientation were obtained at 450 °C using the pressure of 5 × 10−4 Torr, and laser energy of 3.5–4.0 J/cm2. The epitaxial relationship, determined by 2θ−θ and φ scan of XRD and confirmed by transmission electron microscopy (TEM) diffraction patterns, was shown to be rutile(100)||sapphire(0001), rutile[001]||sapphire[10 $\bar 1$0] and rutile[010]||sapphire[1
$\bar 1$0] and rutile[010]||sapphire[1 $\bar 2$10]. An atomically sharp interface between the rutile epitaxial film and the sapphire substrate was observed in the scanning transmission electron microscopy (STEM) images. The films exhibited a transmittance of 75–90% over the visible region. The absorption edge was observed to shift toward longer wave lengths at higher deposition temperatures or lower pressures. X-ray photoelectron spectroscopy and photoluminescence results showed that concentration of lattice point defects, namely oxygen vacancies and titanium interstitials, increased at lower oxygen pressures. Formation of nanostructured films with a surface roughness of -1.5–13.1 nm was confirmed by atomic force microscopy investigations.
$\bar 2$10]. An atomically sharp interface between the rutile epitaxial film and the sapphire substrate was observed in the scanning transmission electron microscopy (STEM) images. The films exhibited a transmittance of 75–90% over the visible region. The absorption edge was observed to shift toward longer wave lengths at higher deposition temperatures or lower pressures. X-ray photoelectron spectroscopy and photoluminescence results showed that concentration of lattice point defects, namely oxygen vacancies and titanium interstitials, increased at lower oxygen pressures. Formation of nanostructured films with a surface roughness of -1.5–13.1 nm was confirmed by atomic force microscopy investigations.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 13: FOCUS ISSUE: Frontiers in Thin-Film Epitaxy and Nanostructured Materials , 14 July 2013 , pp. 1669 - 1679
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 10
- Cited by




