Introduction
Excitotoxic injury, oxygen free radicals, inflammation, necrosis and apoptosis are all thought to play key roles in the pathogenesis of ischemic brain damage.Reference Ankarcrona, Dypbukt and Bonfoco 1 - Reference Liu, Yang, Wang, Liu, Li and Li 5 There is a lack of effective strategies to protect brain cells from these ischemic injury-associated reactive cellular cascades.Reference Leker, Gai, Mechoulam and Ovadia 6 Tissue plasminogen activator (t-PA) thrombolysis is the only protocol for the acute treatment of ischemic stroke that is approved by the United States Food and Drug Administration.Reference Ly, Zavala and Donnan 7 , Reference Lapchak and Zivin 8 However, t-PA thrombolysis therapy can increase vascular permeability, neurotoxicity, neuronal cell damage and hemorrhage.Reference Wu 9 - Reference Yepes, Sandkvist and Wong 15 Furthermore, there is only a 4.5-hour time window to initiate t-PA thrombolytic therapy after the onset of ischemic stroke symptoms.Reference Lees, Bluhmki and von Kummer 16 These limitations result in only 2% of all ischemic stroke patients benefiting from t-PA thrombolysis.Reference Wang, Kilic and Kilic 17 - Reference Broderick 20
It is recommended that neuroprotective agents be used with t-PA thrombolytic therapy for maximal benefits.Reference Ly, Zavala and Donnan 7 , Reference Broderick 20 - Reference Hinkle and Bowman 22 In addition to rescuing nervous tissue from the cellular and molecular pathological events of the ischemic injury, neuroprotective agents may also counteract the adverse effects of t-PA on neurons and other cell types in the brain.Reference Manoonkitiwongsa 23 However, successful experimental treatments in cells and rodents have shown limited efficacy for human ischemic brain damage. Because most tested drugs are active against only one of the damaging processes associated with stroke, other mechanisms may cause cellular death.Reference Leker, Gai, Mechoulam and Ovadia 6 Thus, a combination of protective agents targeting different pathophysiological mechanisms may obtain better effects than a single agent.
Vascular endothelial growth factor (VEGF) is an angiogenic peptide that also exerts diverse neuronal effects in vitro and in vivo, both in the central and peripheral nervous systems.Reference Lazarovici, Marcinkiewicz and Lelkes 24 Exogenous VEGF stimulates neurogenesis and synaptic plasticity, promotes the growth of neurons and glial cells, increases cerebral blood flow and protects neuronal tissues from cell death induced by hypoxia or ischemia.Reference Zhu, Mao and Zhao 25 - Reference Zhang, Zhang and Jiang 31 Nerve growth factor (NGF), another growth factor, can promote the survival of specific groups of neurons both in vivo and in vitro,Reference Shigeno, Mima and Takakura 32 - Reference Vantini, Schiavo and Martino 38 and has protective effects against neuronal death and apoptosis after cerebral ischemia. In our previous study, we found that monotherapy with VEGF and NGF provided significant neuroprotection in the therapeutic time window, which was, respectively, limited within 3 and 5 hours after focal cerebral ischemia.Reference Yang, Guo, Liu and Liu 39 , Reference Yang, Liu, Yang and Feng 40
The major objective of this study was to investigate the effect and therapeutic time window of combination therapy with VEGF and NGF after controlled ischemic brain injury in rabbits.
Materials and Methods
Animals
A total of 64 male New Zealand white rabbits weighing 2.5-3.0 kg were used. The rabbits were housed in separated cages and the room was kept at 24±1°C and 50%-60% humidity, under a 12:12-hour light/dark cycle and with access to food and water ad libitum. All experimental procedures were approved by the local animal care committee and carried out in accordance with the guidelines of the National Institutes of Health on animal care and the ethical guidelines for the investigation of experimental pain in conscious animals. Anesthesia was induced with an intravenous injection of 20 mg/kg pentobarbital sodium and, if necessary, maintained with a further injection of 5 mg/kg. PE-50 polyethylene tubing was inserted into the left femoral artery to monitor arterial blood gases, serum glucose and body temperature before, during and after the operation. For pain relief, the surgical wounds were anesthetized in advance using 2% lidocaine (0.1 ml).
Surgery
Middle Cerebral Artery Occlusion (MCAO)
The intraluminal suture model was used for the induction of focal cerebral ischemia, according to previously described methods.Reference Yang, Liu and Liu 41 , Reference Yang, Liu, Liu, Xu and Ma 42 Briefly, the left common carotid, internal carotid and external carotid arteries were exposed by a midline incision in the neck. Then, the left common and external carotid arteries were ligated proximally (near the bifurcation) with 4-0 surgical sutures. A guide wire (RF SP26137M, Terumo, Tokyo, Japan), with a diameter of 0.53 mm, was inserted into the internal carotid artery from the distal common carotid artery until the tip occluded the origin of the middle cerebral artery (MCA). The placement of the guide wire in the MCA was confirmed using contact X-ray. The guide wire was maintained in place for 2 hours and then withdrawn to allow reperfusion. MRI measurements were performed in all rabbits between 1.75 and 2 hours after MCAO. Animals subjected to the same surgery without vascular occlusion served as the sham-operated group (n=8).
Study Design
Rabbits received intracerebral microinjections of 50 μl of VEGF 165 (2.5 ng/μl) and NGF (16 µg/L)Reference Yang, Liu, Liu, Xu and Ma 42 at different time points (5 or 8 hours post MCAO; n=20 per time point) via the perifocal region. The two specified time points of administration (5 or 8 hours post MCAO) were greater than or equal to the existing therapeutic time window for monoterapy with VEGF or NGF alone (VEGF: 3 hours post MCAO,Reference Yang, Guo, Liu and Liu 39 NGF: 5 hours post MCAOReference Yang, Liu, Yang and Feng 40 ). Control animals received an injection of saline (n=16) at 2 hours after the onset of MCAO. After 2 hours of MCAO and 70 hours of reperfusion, neurological deficits, infarct volume, water content, neural cell apoptosis and the expression levels of caspase-3 and Bcl-2 were measured.
MRI Protocol
Diffusion-weighted imaging was conducted to document injury, to provide a guide for intracerebral injection and to dissect the tissues from the ischemic regions. All animals were imaged in a 1.5-T scanner (Toshiba Visart, Tokyo, Japan), with a quadrature knee coil. Diffusion-weighted imaging was performed using a spin-echo echo-planar imaging sequence (repetition time [TR]=12000 ms, echo time [TE]=108 ms, number of averages [NA]=3, 2 different b-values [b=0 and b=900 s/mm2], field of view [FOV]=16.5 cm×16.5 cm, matrix=96×96, five slices, slice thickness=5 mm). If hyperintensity was observed in the MCA territory, apparent diffusion coefficient (ADC) maps were constructed by acquiring a set of five images with increasing diffusion gradient amplitude. The ischemic core and penumbra regions were performed according to previously described methods.Reference Manabat, Han and Wendland 43 The ranges of the lowest ADC values and the ADC values in the matching contralateral anatomic regions were derived from the ADC maps. The region with ADC values lower than the mean of the lowest ADC values plus 1 SD was referred to as the ischemic core. The region with ADC values higher than the mean of the ADC values plus 1 SD but lower than the mean of the normal ADC values minus 2 SDs in that region was referred to as the ischemic penumbra.
Administration
After the MRI examination, animals were placed in a stereotaxic frame and a burr hole (<2 mm) was made at the left parietal skull, 3 mm posterior from the infraorbital margin and 10 mm lateral to the midline until the dura mater. A single intracerebral microinjection of 50 μl of VEGF 165 (2.5 ng/μl) and NGF (16 µg/L)Reference Yang, Liu, Liu, Xu and Ma 42 was administered directly into the left ischemic penumbra cortex over a 10-minute period at a depth of 6 mm from the skull surface using a stereotactic micromanipulator. The needle was retained in place for 5 minute after each injection. The same volume of saline was administered to the saline-treated group. After the injection, the skull defect was covered with bone wax and then the skin was sutured.
Neurological Evaluation
Animals were examined for neurological function 24 and 72 hours after the onset of occlusion. The neurological findings were scored according to the Purdy scoring method.Reference Purdy, Devous, Batjer, White, Meyer and Samson 44 Each animal was examined for motor function (score=4), consciousness (score=4), head turning (score=1), circling (score=1) and hemianopsia (score=1). The lowest possible score was 2 points, suggesting no neurological impairment, and the highest possible score was 11 points, suggesting that the animals lost consciousness or died.
2,3,5-Triphenyltetrazolium Chloride (TTC) Staining
For the quantitative infarct volume, rabbits were killed at 72 hours of MCAO with an overdose of barbiturates. After the skulls were removed, the brain was rapidly removed and cooled in cold saline for 10 minutes. The brains were then coronally sectioned into five 5-mm-thick sections. The brain slices were incubated for 30 minutes in a 2% solution of TTC at 37°C, and then fixed by immersing in a 4% buffered formalin solution. Infarct size was quantified using an image-processing software package (Beihang University, Beijing, China). To compensate for the effect of brain edema, the infarct volume was measured by a commonly used indirect methodReference Hsiao, Lin and Chang 45 as follows: infarct volume=contralateral hemisphere volume−volume of the intact region in ipsilateral hemisphere.
Brain Water Content
At 72 hours after MCAO, rabbits were killed and the brains were removed. The pons and olfactory bulb were removed and the brains were weighed to obtain their wet weight (ww). Thereafter, the brains were dried at 110°C for 24 hours to determine their dry weight (dw). Brain water content was calculated using the following formula: (ww−dw)/ww×100 and was used as an index for brain edema.Reference Vakili, Kataoka and Plesnila 46
Flow Cytometry Analysis (Percentage of Apoptotic Cells)
Flow cytometry was used to determine the extent of DNA fragmentation in cells extracted from the penumbral cortex. In separate rabbits (n=5 per NGF+VEGF-treated group, n=4 saline-treated group, n=2 sham-operated group), cortical tissue from the penumbral cortex was dissected immediately after killing the animals. The cells were dissociated by repeated aspiration in saline using a glass micropipette. Thereafter, flow cytometry was used to calculate the cell apoptotic rate, according to previously described methods.Reference Yang, Liu, Liu, Xu and Ma 42 , Reference Linnik, Miller and Sprinkle-Cavallo 47 The absorbance ratio was measured at 488 nm.
Immunohistochemistry
For the histological examination, a total of 16 male New Zealand white rabbits (n=5 per NGF+VEGF-treated group, n=4 saline-treated group, n=2 sham-operated group) were anesthetized with pentobarbital sodium and then transcardially perfused with phosphate-buffered 4% paraformaldehyde. In total, five coronal paraffin sections (6-μm-thick each) per animal, corresponding to the MR images, were cut and processed for immunohistochemistry. The sections were washed in phosphate buffered solution (PBS), incubated in 3% H2O2 in PBS for 10 minutes, followed by incubation in the blocking solution (10% goat serum in PBS) for 30 minutes at room temperature and rabbit polyclonal anti-caspase-3 (SC-7272) or anti-Bcl-2 (SC-7382, Wuhan Boshide Inc., Wuhan, China, 1:75) at 4°C overnight. The sections were washed and incubated. The reaction was stopped with diaminobenzidine (DAB). The mean optical density values of the positive expression levels of caspase-3 and Bcl-2 of five non-replicated fields were measured using the Meta Morph microimage analysis system (Molecular Devices, Sunnyvale, CA, USA) under a 400-fold light microscope.
TUNEL Assay
Neuronal damage was assessed by histological analysis of brain sections at 72 hours after MCAO. The number of TUNEL-labeled cells in the penumbral cortex was counted on a computer screen grid from at least five random fields (400×) from each animal. The results are expressed as the average number of cells per field.
Statistical Analyses
All measurements in this study were performed blindly. The results are expressed as the mean±standard deviation. The histological outcome measures were compared using a one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. Non-parametric ANOVA on ranks was used to compare the total neurological scores among the groups. The results were considered significant at p<0.05.
Results
Infarct Volume
A total of 16 rabbits (n=5 per NGF+VEGF-treated group, n=4 saline-treated group, n=2 sham-operated group) were used for the TTC analysis. No cerebral infarct was seen in the sham-operated rabbits. At 72 hours after MCAO, compared with the saline-treated group, the combination therapy with VEGF and NGF significantly reduced the infarct volume when administered 5 or 8 hours after MCAO (p<0.01, Figure 1), and the administration at 5 hours post MCAO performed better (p<0.01).
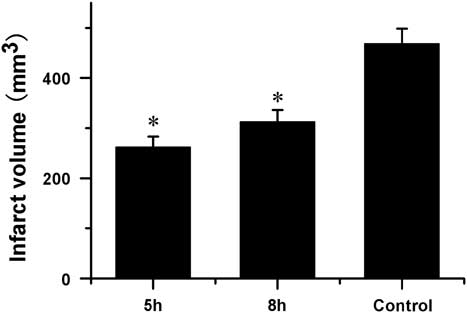
Figure 1 Quantitative effects of combination therapy with vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) on infarct volume after middle cerebral artery occlusion (MCAO) (mean±SD). At 72 hours of MCAO compared to saline-treated group combination therapy with VEGF and NGF at 5 and 8 hours after MCAO significantly reduced infarct volume. *Saline-treated control group p<0.01.
Neurological Deficits
No neurological deficits were seen in the sham-operated rabbits. When tested at 24 or 72 hours after MCAO, the saline-treated rabbits displayed severe neurological deficits. The combination therapy of VEGF and NGF produced significant improvement in neurological scores compared with those in the saline-treated controls when administered 5 or 8 hours post MCAO (Table 1, p<0.01), and the administration at 5 hours post MCAO was more effective (p<0.01).
Table 1 Neurological deficit scores in the vascular endothelial growth factor+nerve growth factor (VEGF+NGF)-treated and saline-treated rabbits (mean±SD)
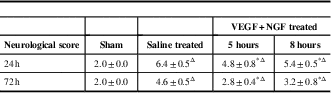
* p<0.01, the saline-treated controls. Δ p<0.01, the sham group.
Brain Water Contents
At 72 hours after MCAO, a significant reduction in water content compared with that in the saline-treated group [(79.2±0.5)%, (79.9±0.6)% vs. (81.8±0.3)%, both p<0.01] was found when the combination therapy was administered 5 or 8 hours after MCAO.
Flow Cytometry Analysis Showing the Percentage of Apoptotic Cells
Apoptosis was rare in the sham-operated group. At 72 hours after MCAO, the percentage of apoptotic cells in the penumbral cortex was markedly increased in the saline-treated group compared with that in the sham-operated group (p<0.01). The percentage of apoptotic cells in the penumbral cortex was significantly reduced in the VEGF+NGF-treated group compared with that in the saline-treated group [(10.4±0.7)%, (15.5±1.2)% vs. (20.2±1.3)%, both p<0.01] when administered 5 or 8 hours post MCAO, and the administration at 5 hours post MCAO had more obvious results (p<0.01).
TUNEL-Positive Cells
A total of 16 rabbits (n=5 per NGF+VEGF-treated group, n=4 saline-treated group, n=2 sham-operated group) were used for TUNEL staining. TUNEL-positive cells were rarely observed in the sham-operated group. At 2 hours of MCAO and 70 hours of reperfusion, the number of TUNEL-positive cells in the penumbral cortex was significantly decreased in the group treated with the combination therapy of NGF and VEGF (5 or 8 hours post MCAO) compared with that in the saline-treated group (7.6±1.5, 11.0±2.9 vs. 32.8±2.6, both p<0.01), and the administration at 5 hours post MCAO had more obvious results (p<0.01).
The Expression of Caspase-3 and Bcl-2
In the sham-operated group, weak caspase-3 immunoreactivity was detected. The expression of caspase-3 in the penumbral cortex was markedly increased in the saline-treated rabbits. Treatment with VEGF and NGF 5 or 8 hours post MCAO produced a significant reduction in the expression of caspase-3 compared with that in the saline-treated control group (p<0.01, Figure 2).
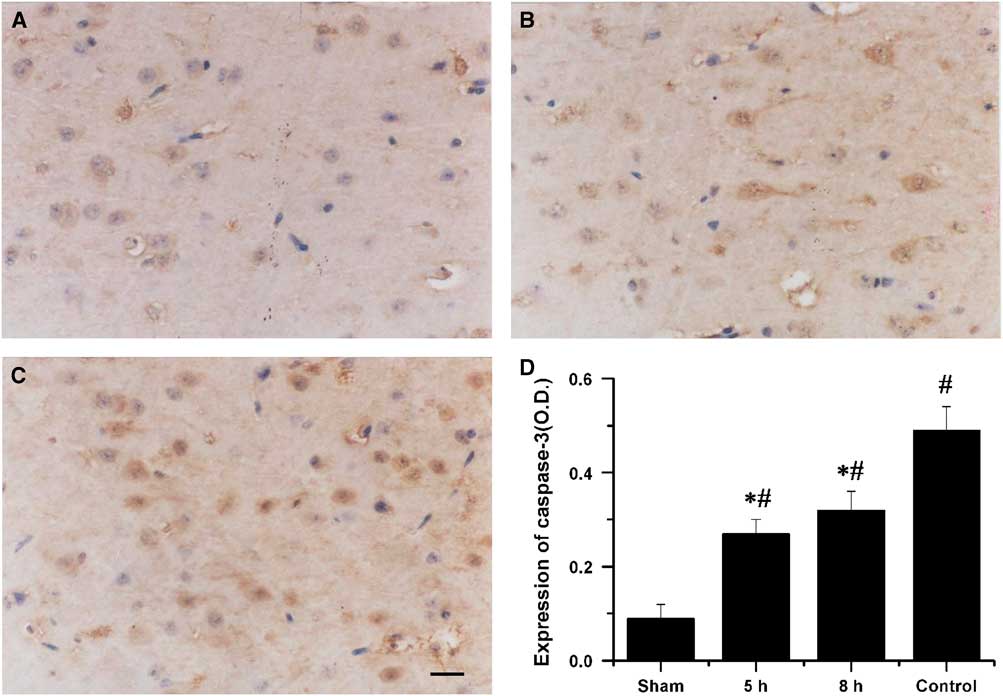
Figure 2 Immunohistochemical staining for caspase-3 in the penumbral cortex after 72 h of middle cerebral artery occlusion (MCAO). The photomicrographs showed caspase-3 expression in the penumbral cortex of animals that received combination therapy of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor at 5 hours (A) and 8 hours (B) after MCAO and saline (C). Bar=20 µm. (D) Quantification of caspase-3 expression in the penumbral cortex after 72 hours of MCAO. *Saline-treated control group p<0.01. #Sham group p<0.01.
In the sham-operated group, no Bcl-2 immunoreactivity was detected in the cerebral cortex. The expression of Bcl-2 in the penumbral cortex was markedly reduced in the saline-treated rabbits. Treatment with VEGF and NGF 5 or 8 hours post MCAO significantly increased the expression of Bcl-2 compared with that in the saline-treated controls (p<0.01, Figure 3).
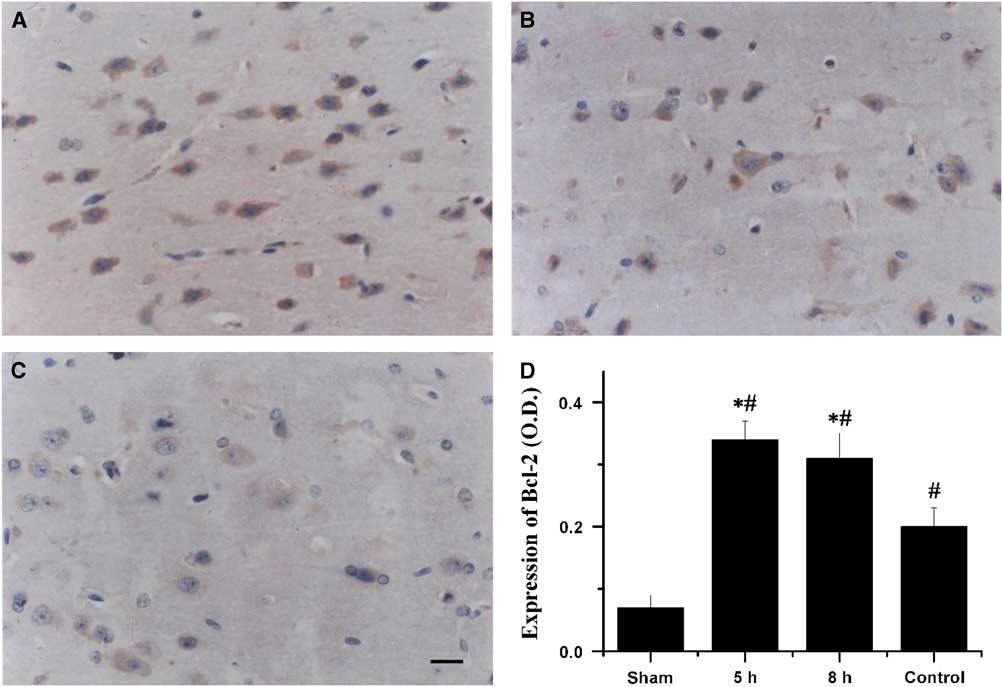
Figure 3 Immunohistochemical staining for Bcl-2 in the penumbral cortex after 72 hours of middle cerebral artery occlusion (MCAO). The photomicrographs showed Bcl-2 expression in the penumbral cortex of animals that received combination therapy of vascular endothelial growth factor and vascular endothelial growth factor at 5 hours (A) and 8 hours (B) after MCAO and saline (C). Bar=20 µm. (D) Quantification of Bcl-2 expression in the penumbral cortex after 72 hours of MCAO. *Saline-treated control group p<0.01. #Sham group p<0.01.
Treatment with VEGF and NGF 5 hours post MCAO showed better results in reducing caspase-3 level and increasing Bcl-2 level.
Discussion
A series of studies have documented that VEGF is up-regulated in the ischemic brain after stroke, and exogenous VEGF is strongly angiogenic and neuroprotective.Reference Zhu, Mao and Zhao 25 - Reference Zhang, Zhang and Jiang 31 Nerve growth factor can promote the survival of specific groups of neurons both in vivo and in vitro,Reference Shigeno, Mima and Takakura 32 - Reference Vantini, Schiavo and Martino 38 and has protective effects against neuronal death and apoptosis after cerebral ischemia. In the present study, combining these two promising growth factors produced significant neuroprotection in focal cerebral ischemia, as evidenced by significant reductions in cerebral infarction, water content, ADC ratio and neurological deficits. The administration of the combination therapy of NGF and VEGF even up to 8 hours post MCAO produced significant reductions in infarct volume, water content and neurological deficits. However, the administration at 5 hours post MCAO performed better. Therefore, the time window of combination treatment with VEGF and NGF should be at least 8 hours after MCAO. The time point (8 hours post MCAO) was greater than the existing therapeutic time window for monotherapy with VEGF or NGF alone, which was, respectively, limited within 3 or 5 hours after focal cerebral ischemia.Reference Yang, Guo, Liu and Liu 39 , Reference Yang, Liu, Yang and Feng 40 Thus, we concluded that the therapeutic time window for the administration of the combined therapy of NGF and VEGF in the rabbit focal cerebral ischemia model should be longer than that for monotherapy with NGF or VEGF alone. This finding implies that the combination therapy of NGF and VEGF might be of clinical value for the treatment of stroke.
Apoptosis is an important form of cell death and contributes to the development of neuronal ischemic infarction after ischemic injury.Reference Yang, Liu, Liu, Xu and Ma 42 , Reference Nitatori, Sato and Waguri 48 , Reference Li, Sharov, Jiang, Zaloga, Sabbah and Chopp 49 Caspase-3 accelerates apoptosis and has been suggested as an apoptotic marker.Reference Lee, Zipfel and Choi 50 , Reference Schulz, Weller and Moskowitz 51 Bcl-2 provides protection against apoptosis by inhibiting cytochrome c translocation, thereby blocking caspase-3 activation and the apoptotic process.Reference Shimizu, Narita and Tsujimoto 52 , Reference Yang, Liu and Bhalla 53 This study showed that combination therapy with NGF and VEGF inhibited the expression of pro-apoptotic proteins (caspase-3) and induced the expression of the anti-apoptotic protein Bcl-2 in a rabbit stroke model and treatment 5 hours post MCAO showed the better results, thereby providing the molecular evidence for its neuroprotective activity. In addition, combination therapy with NGF and VEGF showed clear neuroprotective and anti-apoptotic activity in a rabbit stroke model. These results suggest that combination therapy with NGF and VEGF may exert therapeutic effects on stroke by directly modulating cellular apoptotic processes.
In summary, these studies showed that combination therapy with VEGF and NGF may provide neuroprotective effects, including significant inhibition of neuronal cell apoptosis and improvement in functional recovery. Furthermore, we concluded that the time window of combination treatment with VEGF and NGF should be at least 8 hours after MCAO, which was wider than monotherapy with VEGF and NGF alone in ischemia stroke.
Acknowledgments
This study was supported by the Hebei Natural Science Foundation (H2014206139, J Yang).
Statement of Authorship
JY, BY, BX, JQ, and HL participated in the conception, design, data analysis, interpretation, drafting and the critical revising for important intellectual content of this manuscript, and approved the final version. BY, BX, and JQ participated in the data collection.
Disclosures
JY reports grants from Hebei Natural Science Foundation, during the study period. BY, BX, JQ, and HL have nothing to disclose.








