Vitamin B12 (B12) is involved in DNA synthesis, methylation of biomolecules, and neuron myelination, which are crucial processes for healthy fetal development and growth( Reference Dror and Allen 1 ). Maternal B12 status during pregnancy has been inversely associated with a wide array of adverse health outcomes for mother and offspring, including neural tube defects( Reference Molloy, Kirke and Troendle 2 – Reference Ratan, Rattan and Pandey 4 ), restricted fetal growth( Reference Muthayya, Kurpad and Duggan 5 – Reference Rogne, Tielemans and Chong 7 ), and offspring predisposition for non-communicable diseases( Reference Stewart, Christian and Schulze 8 – Reference Krikke, Grooten and Vrijkotte 10 ). Notably, South Asians have been described to have an increased risk for such adverse pregnancy outcomes, including neural tube defects( Reference Ratan, Rattan and Pandey 4 ) as well as a fetal anthropometry associated with cardiometabolic diseases( Reference Yajnik, Fall and Coyaji 11 – Reference Stanfield, Wells and Fewtrell 13 ). Studies from the Indian subcontinent showed low maternal B12 status to be related to an increased risk of intra-uterine growth retardation( Reference Muthayya, Kurpad and Duggan 5 , Reference Duggan, Srinivasan and Thomas 6 ), low birth weight( Reference Rogne, Tielemans and Chong 7 ) and offspring insulin resistance( Reference Stewart, Christian and Schulze 8 , Reference Yajnik, Deshpande and Jackson 9 ). South Asians are the largest ethnic minority in Canada( 14 ) and the UK( 15 ), and one of the largest growing ethnic minorities in the USA( Reference Hoeffel, Rastogi and Kim 16 ). As such, it is imperative to investigate B12 status in South Asian pregnant women in these countries.
Some populations and ethnic groups, including South Asians, with low intakes of animal-source foods are at increased risk for inadequate B12 status( Reference Allen 17 – Reference Jiang, Christian and Khatry 19 ). Prevalence of B12 deficiency in non-pregnant South Asian populations in Canada, the USA and the UK was reported to be higher compared with that in population groups of European or other ethnicities in a limited number of smaller studies( Reference Carmel, Mallidi and Vinarskiy 20 – Reference Chackathayil, Patel and Gill 23 ). Logistic challenges and cultural barriers have been identified limiting health research in South Asians( Reference Quay, Frimer and Janssen 24 ). The prevalence of B12 deficiency, defined as serum total B12 concentration<148 pmol/l, among reproductive-aged and pregnant women in Canada( Reference MacFarlane and Greene-Finestone 25 – Reference Jeruszka-Bielak, Isman and Schroder 30 ) and the UK( Reference Sukumar, Adaikalakoteswari and Venkataraman 31 ) was reported to range between 5 and 40 %. A recent cross-sectional study in pregnant women (20–35 weeks of gestation) residing in Vancouver, Canada, in which 18 % of the total sample had a total B12 concentration<148 pmol/l, revealed a markedly higher prevalence among women whose self-identified ethnicity was South Asian (61·5 % (n 16/26)) compared with European (16 % (n 24/150))( Reference Jeruszka-Bielak, Isman and Schroder 30 ). Taken together, B12 status appears to be low in Canadian pregnant women and South Asians are vulnerable to B12 deficiency; yet, larger studies addressing ethnic differences are needed to confirm our preliminary findings on B12 status in South Asian pregnant women living in Canada.
Multiple biomarkers are available to assess B12 status and no consensus has yet been reached on a valid assessment of B12 deficiency in pregnant women. Total B12 is the most commonly used direct B12 biomarker; however, it has been criticised for its limited sensitivity( Reference Hannibal, Lysne and Bjørke-Monsen 32 ). Further, total B12 concentration decreases throughout gestation( Reference Murphy, Molloy and Ueland 33 ) leading to a potential misclassification of B12 status using non-pregnant cutoffs. Holotranscobalamin (holoTC) is the fraction of circulating B12 that is bioavailable to tissues including the placenta( Reference Quadros, Nakayama and Sequeira 34 ). Circulating holoTC concentrations have been suggested to remain largely unchanged during pregnancy( Reference Mørkbak, Hvas and Milman 35 , Reference Greibe, Andreasen and Lildballe 36 ). As such, holoTC may provide a more meaningful and accurate direct measure of B12 status in pregnant women. Current recommendations advise the use of one direct and one functional biomarker for B12 status assessment( Reference Yetley, Pfeiffer and Phinney 37 ). The functional B12 biomarkers are methylmalonic acid (MMA) and total homocysteine (tHcy). MMA is considered the more specific functional biomarker, because tHcy is determined by other B-vitamins in addition to B12. Yet, elevated tHcy concentration has been associated with adverse pregnancy outcomes, independent of B-vitamin status( Reference Furness, Fenech and Dekker 38 ). MMA is confounded by renal insufficiency( Reference Rasmussen, Vyberg and Pedersen 39 ) as is tHcy( Reference Stabler, Marcell and Podell 40 ). The ratio of 2-methylcitric acid (MCA):MMA may be used to assess renal function when creatinine concentrations are not available, because MCA concentrations were shown to be substantially higher than MMA concentrations, that is, MCA>MMA, in patients with renal failure compared with those with normal renal function( Reference Allen, Stabler and Savage 41 ). Given the limitations of each biomarker, quantification of all four biomarkers allows for the most comprehensive assessment of B12 status.
Maternal B12 adequacy is critical for maternal health and optimal fetal growth and development. In light of South Asians being the largest ethnic minority in Canada and the higher risk of B12 deficiency for this ethnic minority, we aimed to assess B12 status, using multiple biomarkers, in pregnant women of South Asian and, for comparison purposes, European ethnicity, residing in Vancouver, Canada.
Methods
Study design and setting
This retrospective cohort study utilised residual serum samples, which had been routinely collected during prenatal genetic screening in British Columbia (BC), Canada (BC Prenatal Genetic Screening Program( 42 )). The programme is offered free of charge and to all pregnant women residing in BC (Canada)( Reference Metcalfe, Lix and Johnson 43 ). Participation is entirely voluntary and ranges from 38·5 % among pregnant women aged 20–24 years to 68·8 % among those aged 35–39 years( 44 ). The screening involves non-fasting blood collections at two time points: approximately 11 (range 9–13) gestational weeks (1st trimester) and 17 (range 15–19) gestational weeks (2nd trimester). Demographic and other health-related information are recorded during the blood collection.
The University of British Columbia Children’s and Women’s Research Ethics board (Vancouver, Canada) reviewed this study and approved a waiver of individual consent for the secondary use of deidentified clinical samples from the BC Prenatal Genetic Screening Program (institutional approval no.: H15-00820).
Study sample and data collection
Serum samples were included in the study if the women had identified themselves during the registration process for the BC Prenatal Genetic Screening Program as being either of ‘South Asian’ or ‘Caucasian’ (i.e. European origin and, for clarity, from here forth referred to as European) ethnicity and were aged 19–45 years old. In addition, only samples collected within the Lower Mainland (Metro Vancouver, BC, Canada) were retrieved for the study. Samples of women who had a multiple gestation, a pregnancy after in vitro fertilisation or steroid use, diabetes mellitus (I/II), history of smoking, or a positive screen for a chromosomal abnormality or an open neural tube defect in this or a past pregnancy were excluded from the study. Information on maternal age, self-identified ethnicity and gestational week at blood collection (estimated by fetal ultrasound crown-rump-length) was obtained from medical charts completed during the prenatal screening visit. The serum samples retrieved for this study were collected between November 2014 and May 2016.
Maternal non-fasting blood samples were collected into serum separator tubes and left at room temperature for serum to separate, following standard protocol. Samples were subsequently stored at 4°C, centrifuged within 24 h of sample collection at 1890 g for 5 min at 4°C, and transferred to −80°C within 4 d. Samples were thawed once allowing for deidentification. Specifically, samples were thawed on ice, separated into aliquots (one aliquot per biomarker assay) labelled with a unique study identification number (ID), and subsequently stored at −80°C until samples were thawed for specific biomarker analyses. The two freeze-thaw cycles in total for this study will not have affected the B12 biomarker concentrations: serum MMA concentrations are unaffected by up to five freeze-thaw cycles( Reference Pedersen, Keyes and Shahab-Ferdows 45 ), tHcy is not affected by repeated freezing and thawing( Reference Rasmussen and Møller 46 ), total B12 is stable for ten repeated freeze-thaw cycles( Reference Gislefoss, Lauritzen and Langseth 47 ), and up to three freeze-thaw cycles are acceptable for holoTC measurements according to the manual of the assay manufacturer (Abbott Laboratories). Further, the use of non-fasting samples is acceptable for B12 status assessment as B12 biomarker concentrations remain unchanged during the postprandial stage( Reference von Castel-Roberts, Morkbak and Nexo 48 , Reference Rasmussen 49 ).
Laboratory analyses
Serum samples were analysed, sequentially, for the B12 biomarkers total B12, holoTC, MMA with MCA, and tHcy as well as folate between January 2016 and September 2016. If the sample volume was insufficient for an aliquot, the analyte was omitted in the respective sample. Samples were analysed at random to avoid analytical bias between ethnicities.
Serum total B12 and holoTC were quantified by fully automated immunoassays (Access 2 by Beckman Coulter and Architect by Abbott Laboratories, respectively) according to manufacturers’ protocols at the pathology laboratories at BC Children’s Hospital and St. Paul’s Hospital, respectively. The inter-assay CV for four serum total B12 control samples (Bio-Rad, mean concentration of 93·6, 245, 335, 407 pmol/l) ranged from 2·4 to 7·1 % (analysed weekly over 4 months; n 18). Manufacturer (Abbott Laboratories) control samples for holoTC were run once per batch for the three batches of study samples, yielding mean concentrations of 46 (sd 1·5) pmol/l (CV: 3·3 %) and 16 (sd 2·0) pmol/l (CV: 12 %) for the high and low control sample, respectively. The upper limits of the analytical measurement range for the total B12 and holoTC assays were 1107 and 128 pmol/l, respectively. Due to the limited volume of residual serum samples, repeated analyses of samples with total B12 and holoTC concentrations above the upper limit of the measurement range were not possible.
MMA in serum was determined by stable isotope dilution-liquid chromatography-tandem MS (LC-MS/MS) as previously described( Reference Schroder, Quay and Lamers 50 ). Simultaneously with MMA, MCA was quantified using MCA (Sigma-Aldrich) as standard and d3-MMA (Cambridge Isotopes Ltd) as internal standard. Results are reported as sum of all stereoisomers. MCA>MMA was used as an indicator of renal insufficiency( Reference Allen, Stabler and Savage 41 ). Although MCA>MMA has not yet been confirmed as an indicator of renal insufficiency in pregnancy, one woman was excluded from the analyses on this basis. The inter-assay CV for an in-house control sample was 7 % for MMA and 14 % for MCA (analysed over 19 d); the intra-assay CV was<5 % for all analyses.
Serum tHcy was quantified using stable isotope dilution LC-MS/MS. In brief, after reduction with dithiothreitol and protein precipitation, samples were injected into an LC system (Agilent 1260; Agilent Technologies). Compounds were separated by a normal phase column (Fortis H2O, 2·1×150 mm, 5 µm; Fortis Technologies). The mobile phase consisted of (A) 0·2 % heptafluorobutyric acid in water and (B) 0·2 % heptafluorobutyric acid in acetonitrile using a gradient run (A:B 95:5 (v/v) to 20:80 (v/v)). The affluent was directed into an MS/MS system (API4000; SCIEX Pte). Quantification was performed with seven-point calibration curves (1·14–114 µmol/l) made using l-homocysteine (Sigma-Aldrich) as calibrator and d4-homocysteine (Cambridge Isotopes Ltd) as internal standard. The inter-assay CV for an in-house control sample was 9·4 % (analysed over 17 d). Two external quality control samples (ClinChek 23082 and IRIS Technologies International) were quantified with every analysis and were within their acceptable range of 9·0 (sd 1·8) and 25·9 (sd 5·1) µmol/l, respectively.
Serum folate was analysed using the microbiological assay according to the method developed by O’Broin & Kelleher( Reference O’Broin and Kelleher 51 ) and Molloy & Scott( Reference Molloy and Scott 52 ). The assay was performed using the chloramphenicol-resistant Lactobacillus rhamnosus (ATCC 27773) and 5-methyltetrahydrofolate ((6S)-5-methyl-5,6,7,8-tetrahydropteroyl-l-glutamic acid, sodium salt; Merck Eprova) as calibrator. Control samples were included in each of the twenty-two batch runs. The analyses yielded folate contents of 28·3 nmol/l (13 ng/ml) (inter-assay CV: 8·8 %) for the NIBSC 95/528 control sample (13 ng/ml) and of 42·8 nmol/l (inter-assay CV 10·1 %) for an in-house serum control sample.
Biomarker cutoffs
To date, there are no established cutoffs to define B12 status in pregnancy for any of the four available B12 biomarkers. Pregnant women were, thus, classified using non-pregnant adult cutoffs to allow for comparison with previous research. It is commonly accepted that serum total B12 concentrations<148 pmol/l( Reference de Benoist 53 ) or serum holoTC concentrations<35 pmol/l( Reference Herrmann, Obeid and Schorr 54 – Reference Brady, Wilson and McGregor 56 ) indicate B12 deficiency in non-pregnant adults. Serum total B12 concentrations<221 pmol/l( Reference Molloy, Kirke and Troendle 2 ) and serum holoTC concentrations<55 pmol/l( Reference Ray, Wyatt and Thompson 3 ) have been associated with an increased risk for neural tube defect-affected pregnancies and will be referred to as inadequate B12 status. Elevated and mildly elevated MMA concentrations were defined as serum MMA concentrations >370( Reference Hølleland, Schneede and Ueland 57 ) and >210 nmol/l( Reference Pfeiffer, Caudill and Gunter 58 ), respectively. Overt B12 deficiency was defined as combined serum total B12 concentrations<148 pmol/l and serum MMA concentrations >370 nmol/l.
Serum tHcy concentrations >13 µmol/l have been used to define elevated tHcy concentrations in pregnant women and populations with folic acid fortification( Reference Visentin, Masih and Plumptre 26 , Reference Pfeiffer, Caudill and Gunter 58 – Reference van Meurs, Dhonukshe-Rutten and Pluijm 60 ). In Canada, mandatory fortification of flour with folic acid has been in place since 1998( Reference Colapinto, O’Connor and Dubois 61 ). In addition, tHcy concentrations<9 µmol/l have been described as ‘desired’( Reference Pfeiffer, Caudill and Gunter 58 ). Folate deficiency and inadequate folate status were defined as having serum folate concentration<6·8 and<13·4 nmol/l, respectively, as proposed by the World Health Organization( 62 ).
Statistical analyses
The study aimed to retrieve sets of two serum samples (1st trimester and 2nd trimester) collected from 600 women of South Asian (n 300) and European (n 300) ethnicity during pregnancy. This sample size allowed for the detection of a difference in serum total B12 concentrations between pregnant women of South Asian and European ethnicity of 40 pmol/l with a power of 0·80 at a confidence level (α) of 0·05. Sampling was continued until sufficient complete sets (1st and 2nd trimester) had been obtained. Normality of data was tested visually (Kernel’s density distribution) and by Shapiro–Wilk test. Non-normally distributed data are presented as geometric means and 95 % CI. Given the censored data (at 128 pmol/l), mean (95 % CI) holoTC concentrations were estimated by Tobit regression after ln-transformation. Differences in biomarker concentrations, maternal age, and gestational week at sample collection between women of South Asian and European ethnicity were determined by Wilcoxon’s rank-sum test; differences in prevalence of B12 deficiency and inadequate B12 status were determined by Pearson’s χ 2 test or likelihood-ratio χ 2 test, when cell size<5 (i.e. for tHcy and folate). Cohen’s d was used to estimate the effect size of the difference in biomarker concentrations between women of South Asian and European ethnicity. The association between maternal B12 status and ethnicity was tested by three mixed-effects generalised linear models. In these models, either maternal serum total B12 (ln-transformed), serum holoTC (ln-transformed and 133 (20 %) and 90 (14 %) participants with serum holoTC ≥128 pmol/l during 1st and 2nd trimester, respectively, excluded), or serum MMA (ln-transformed) concentration served as the dependent variable and maternal ethnicity (South Asian), maternal age (years), and gestational week at sample collection (weeks) as independent variables. The models accounted for repeated measures by including the study ID as a random effect to account for autocorrelation among samples from the same person. All statistical analyses were performed in Stata 14.2 (StataCorp LP) for Windows 10 (Microsoft Corp.), with the level of significance set at P<0·05.
Results
To meet the sampling goal of the study, samples and data of 748 women, aged 19–44 years, were retrieved for this study. The mean gestational week at the two study time points was 11·5 (range 8·3–13·9) weeks for the 1st trimester and 16·5 (range 14·9–20·9) weeks for the 2nd trimester (Table 1). There were no significant differences in gestational weeks at sample collection between pregnant women of South Asian and European ethnicity. Further, there were no significant differences in maternal characteristics between pregnant women of South Asian and European ethnicity after exclusion of women with missing data for total B12, holoTC, MMA, tHcy or folate (data not shown).
Table 1 Maternal characteristicsFootnote * (Mean values and ranges)

* Maternal ethnicity, that is, European or South Asian, was self-reported. P value is reported for the difference between pregnant women of European and South Asian ethnicity as determined by Wilcoxon’s rank-sum test.
Vitamin B12 biomarker concentrations were quantified in at least 300 samples from women of each South Asian and European ethnicity (in total n 600) per time point, that is, the calculated target sample size, except for tHcy concentrations (Fig. 1). Missing data, for example tHcy concentrations, resulted from insufficient sample volume to quantify the respective analyte. The results of B12 biomarker concentrations are summarised in Table 2. Pregnant women of South Asian compared with European ethnicity had significantly lower serum total B12 and serum holoTC concentrations and significantly higher serum MMA concentrations at both time points. The effect size of the difference was largest for 1st and 2nd trimester serum total B12 (Cohen’s d: 0·52 and 0·45, respectively) and smallest for holoTC concentrations (Cohen’s d: 0·28 and 0·20, respectively). There was no difference in serum tHcy and folate concentrations between pregnant women of South Asian and European ethnicity.

Fig. 1 Flow diagram of serum sample collection and biomarker analyses. The sequence of biochemical analyses reflects the prioritisation of biomarker analyses in dependence of available serum volume and successful biomarker quantitation. B12, vitamin B12; HoloTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
Table 2 Serum total vitamin B12 (B12), holotranscobalamin (holoTC), methylmalonic acid (MMA), total homocysteine (tHcy) and folate concentrations of women of European and South Asian ethnicity during 1st and 2nd trimester of pregnancy (Geometric means and 95 % confidence intervals)

Significant differences within 1st or 2nd trimester between pregnant women of European and South Asian ethnicity: * P≤0·01, ** P≤0·001, *** P≤0·0001, as tested by Wilcoxon’s rank-sum test; concentrations determined in non-fasting serum samples.
† Mean (95 % CI) holoTC concentrations were estimated by Tobit regression.
A significantly higher prevalence of women of South Asian compared with European ethnicity were classified as B12 deficient and having inadequate B12 status, respectively, as indicated by serum total B12 (<148 and<221 pmol/l) and serum holoTC (<35 and<55 pmol/l) at either time point in the study (Table 3). The prevalence of women classified as B12 deficient in this study differed substantially depending on biomarker and time point of sample collection. It ranged from 5·3 % (serum holoTC<35 pmol/l, both time points) to 25·4 % (serum total B12<148 pmol/l, 2nd trimester) (Table 3). More than half of all women were classified as having inadequate B12 status, indicated by serum total B12<221 pmol/l at both visits. However, only ≤25 % were classified as having inadequate B12 status at both visits when using serum holoTC<55 pmol/l as indicator. The prevalence of women with elevated serum MMA concentrations (>370 nmol/l) and mildly elevated serum MMA concentrations (>210 nmol/l) was approximately 6·5 and 12 %, respectively, at both time points. This indication of functional B12 deficiency was not reflected in elevated serum tHcy concentrations (>13 or 9 µmol/l, approximately 0 %). During the 1st and 2nd trimester, 4·8 % (32/669) and 4·9 % (34/697) of all women, respectively, were classified as having overt B12 deficiency (total B12<148 pmol/l and MMA >370 pmol/l) with a significantly higher prevalence in women of South Asian ethnicity (8·9 and 8·3 %, respectively) compared with those of European ethnicity (0·9 and 1·4 %, respectively) at both time points (P<0·001). Folate status was adequate in all but one woman of South Asian ethnicity who had inadequate folate status (<13·4 nmol/l) in the 1st trimester.
Table 3 Prevalence of pregnant women of European and South Asian ethnicity classified as vitamin B12 (B12) inadequate or B12 deficient according to cutoffs indicated in the first column (Prevalences (%) and absolute numbers (n))

holoTC, holotranscobalamin; MMA, methylmalonic acid; tHcy, total homocysteine.
Significant differences within the 1st or 2nd trimester between pregnant women of European and South Asian ethnicity: ** P≤0·001, *** P≤0·0001 as tested by Pearson’s χ 2 test (total B12, holoTC, MMA) or likelihood-ratio χ 2 test (tHcy); concentrations determined in non-fasting serum samples.
† B12 inadequate.
‡ B12 deficient.
To examine the association between maternal ethnicity and B12 status, we determined the predictors of maternal serum total B12, holoTC, and MMA concentration (ln-transformed). Being of South Asian ethnicity predicted a 22 % (P<0·0001) and 12 % (P<0·0001) lower mean serum total B12 and holoTC concentration, respectively, and a 21 % (P<0·0001) higher mean serum MMA concentration (Table 4). Gestational week at sample collection was a significant negative predictor of maternal B12 biomarker concentrations (P<0·005), whereas maternal age was not associated with maternal B12 status.
Table 4 Estimates for South Asian ethnicity to predict maternal serum total vitamin B12 (B12), holotranscobalamin (holoTC), and methylmalonic acid (MMA) concentrations during 1st and 2nd trimester, determined using mixed effects models adjusted for maternal age and gestational week at sample collection and performed on ln-transformed data while accounting for repeated measuresFootnote * (Estimates (B) and 95 % confidence intervals)
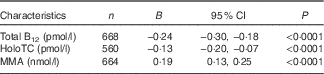
* Exponentiated B describe the factor change of biomarker concentrations when being of South Asian compared with European ethnicity, for example, serum total B12 concentration is multiplied by a mean of e −0·24=0·78 when a pregnant woman is of South Asian compared with European ethnicity and as such being of South Asian ethnicity compared with European ethnicity predicts a 22 % lower mean serum total B12 concentration; P values were determined by likelihood ratio test after adding ethnicity into the models.
Discussion
In this retrospective cohort study of 748 pregnant women residing in Canada, South Asian compared with European women had significantly lower B12 status, as indicated by lower serum total B12 and holoTC concentrations, and higher serum MMA concentrations during early pregnancy. For example, the difference in mean serum total B12 concentration between South Asian and European women in the 1st and 2nd trimester was approximately 23 % (57 and 50 pmol/l, respectively). In addition, South Asian ethnicity was a significant negative predictor of B12 status, as assessed by total B12, holoTC, and MMA concentrations. During the 1st and 2nd trimesters, the prevalence of pregnant women who were classified as overtly B12 deficient (total B12<148 pmol/l and MMA >370 pmol/l) was ten and six times higher, respectively, among South Asian women (who had prevalences of approximately 9 and 8 %, respectively) compared with that of European women (approximately 1 % at both time points). Thus, findings from this study in Vancouver suggest that women of South Asian compared with those of European ethnicity have markedly lower B12 status during the 1st and 2nd trimesters of pregnancy.
The 30 and 36 % prevalence of South Asian pregnant women with total B12 concentration<148 pmol/l in 1st and 2nd trimesters, respectively, in the present study is lower than findings in pregnant women living on the Indian subcontinent. Studies from rural and urban India reported a substantially higher prevalence of approximately 50–70 % of pregnant women having total B12 concentrations<150 pmol/l during early pregnancy( Reference Duggan, Srinivasan and Thomas 6 , Reference Yajnik, Deshpande and Jackson 9 , Reference Katre, Bhat and Lubree 18 ); one study from Nepal found a 28 % prevalence during the 1st trimester( Reference Stewart, Christian and Schulze 8 , Reference Jiang, Christian and Khatry 19 ). In addition, studies from India reported 70–95 % of pregnant women to have MMA concentrations >260 nmol/l( Reference Duggan, Srinivasan and Thomas 6 , Reference Yajnik, Deshpande and Jackson 9 ), which is also substantially higher than the approximately 12 % prevalence of MMA concentrations >260 nmol/l among South Asian pregnant women during either trimester observed in the present study (data not shown). The prevalence of South Asian women with mildly elevated MMA concentrations (>210 nmol/l) in the present study was approximately 20 %. On the other hand, the 10 and 15 % prevalence of European women with total B12 concentrations<148 pmol/l during 1st and 2nd trimester, respectively, was comparable with previous reports of B12 status during early pregnancy in Canada with 5–17 % of predominantly European pregnant women being classified as B12 deficient (total B12<148 pmol/l)( Reference Visentin, Masih and Plumptre 26 , Reference Wu, Innis and Mulder 63 ). A recent cross-sectional study in Vancouver found 16 % of European pregnant women (n 150) to have serum total B12 concentration<148 pmol/l at 20–35 weeks of gestation( Reference Jeruszka-Bielak, Isman and Schroder 30 ). In the present study, 1·5 and 2·5 % of European women had holoTC concentrations<35 pmol/l during 1st and 2nd trimester, respectively. Similarly, the prevalence of holoTC concentrations<35 pmol/l in the Alberta Pregnancy Outcomes and Nutrition study was negligible( Reference Fayyaz, Wang and Jacobs 55 ). Other studies in Canadian pregnant women did not use holoTC as an indicator of B12 status( Reference Visentin, Masih and Plumptre 26 , Reference Jeruszka-Bielak, Isman and Schroder 30 ). As such, whereas B12 biomarker concentrations did not reflect as low a B12 status in South Asian pregnant women in the present study as was reported for women residing on the Indian subcontinent, the prevalence of South Asians classified as B12 deficient was substantial and at least twice as high (depending on biomarker and cutoff) compared with pregnant women of European ethnicity in the present and other Canadian cohorts.
Overall, the prevalence of pregnant women classified as B12 deficient depends on the biomarker and cutoffs used; in the present study, using total B12<148 pmol/l resulted in a prevalence of deficiency at least three-times higher than any other indicator. This tendency was also reflected in the prevalence of pregnant women classified as B12 inadequate. We acknowledge that physiological changes during pregnancy may impact biomarker concentrations independent of B12 status and pregnancy-specific cutoffs are currently lacking. Yet, infants of mothers, living in rural Nepal who had serum total B12 concentrations<148 pmol/l at approximately 11 weeks of gestation (n 147/524), have previously been reported to have a significantly higher (approximately 27 %) estimated insulin resistance using the homeostasis model assessment (HOMA-IR) at age 6–8 years( Reference Stewart, Christian and Schulze 8 ). Further, Indian mothers with total B12 concentration<148 pmol/l during 1st and 2nd trimester (median 115 (interquartile range (IQR) 104, 125) and 112 (IQR 99, 122) pmol/l, respectively) had higher odds (OR 5·98; 95 % CI 1·72, 20·74 and 9·28; 95 % CI 2·90, 29·68, respectively) of intra-uterine growth retardation than mothers with higher total B12 concentrations (median 224 (IQR 206, 268) and 210 (IQR 177, 217) pmol/l, respectively)( Reference Muthayya, Kurpad and Duggan 5 ). In a recent meta-analysis, maternal total B12 concentration<148 pmol/l was related to an increased risk of low birth weight (risk ratio 1·15; 95 % CI 1·01, 1·31) and preterm birth (risk ratio 1·21; 95 % CI 0·99, 1·49)( Reference Rogne, Tielemans and Chong 7 ). Inadequate maternal B12 status (total B12<221 pmol/l or holoTC<55 pmol/l) at <28 d of gestation has been associated with an increased risk for neural tube defects( Reference Molloy, Kirke and Troendle 2 , Reference Ray, Wyatt and Thompson 3 ). Thus, although further research is needed to develop and evaluate pregnancy-specific cutoffs for B12 deficiency, low B12 status and especially total B12 concentrations <148 pmol/l have been associated with adverse pregnancy outcomes in South Asians and other ethnicities.
One of the pregnancy-related changes highlighting the need for pregnancy-specific cutoffs is the decrease in circulating total B12 concentration during healthy pregnancy, which has previously been described( Reference Murphy, Molloy and Ueland 33 , Reference Milman, Byg and Bergholt 59 , Reference Koebnick, Heins and Dagnelie 64 , Reference Hure, Collins and Smith 65 ). It may be due to hemodilution or other physiological changes. In the present study, gestational week at sample collection was a negative predictor of all B12 biomarker concentrations, that is total B12, holoTC, MMA and tHcy. As such, these findings support the hypothesis that circulating B12 biomarker concentrations, especially total B12 concentration, decrease throughout pregnancy, emphasising the need for pregnancy-specific cutoffs for B12 biomarkers.
Low intakes of animal-source foods, low socioeconomic status, and a lower prevalence of supplement use have been suggested as predictors of B12 deficiency in South Asians( Reference Bowes and Domokos 66 – Reference Parackal, Smith and Parnell 68 ). No dietary or demographic data were available in the present retrospective study to determine predictors of the reported differences in B12 status. In addition, some genetic factors may influence B12 biomarker concentrations, independent of B12 intake( Reference Molloy, Pangilinan and Mills 69 ). Variants in the FUT2 gene have been identified as predictors of low serum total B12 concentrations in South Asians( Reference Tanwar, Chand and Kumar 70 ) and have been suggested to explain part of the association between low serum total B12 concentration and obesity( Reference Allin, Friedrich and Pietzner 71 ). An increased prevalence of obesity has been described in South Asian populations, including South Asian pregnant women in the UK( Reference Parackal, Smith and Parnell 68 , Reference Bryant, Santorelli and Lawlor 72 ). Future research is warranted to identify dietary, socioeconomic and genetic predictors of low B12 status in pregnant women, especially in those of South Asian ethnicity, living in Canada to allow for targeted interventions.
None of the women in the present study had folate deficiency and<1 % had tHcy concentration >9 µmol/l at both time points. Folate status in Canadian pregnant women( Reference Fayyaz, Wang and Jacobs 55 , Reference Plumptre, Masih and Ly 73 ) and reproductive-aged women( Reference Colapinto, O’Connor and Dubois 61 ) was reported to be substantially elevated. The high folate status in Canadian pregnant women is explained by the prevalent use of prenatal supplements (>90 %( Reference Fayyaz, Wang and Jacobs 55 , Reference Plumptre, Masih and Ly 73 )) and the high dosage of folic acid (most commonly 1 mg/d) in prenatal supplements available on the Canadian market. Elevated tHcy was not found in Canadian pregnant women( Reference Visentin, Masih and Plumptre 26 ) and was observed in 5 % of the general Canadian population( Reference MacFarlane and Greene-Finestone 25 ). The high folate status in Canadian pregnant women likely explains the low tHcy concentration as described previously( Reference Visentin, Masih and Plumptre 26 ) and found in the present study. Further, pregnant women have lower tHcy concentration compared with non-pregnant women( Reference Murphy, Scott and Arija 74 ), likely due to an increase in remethylation( Reference Kurpad, Anand and Dwarkanath 75 , Reference Dasarathy, Gruca and Bennett 76 ), emphasising the need for pregnancy-specific cutoffs of all B12 biomarkers. This study contributes to the body of evidence that folate status in pregnant women in Canada is high, whereas B12 status may be low( Reference Visentin, Masih and Plumptre 26 , Reference Colapinto, O’Connor and Dubois 61 , Reference Masih, Plumptre and Ly 77 , Reference Shakur, Garriguet and Corey 78 ).
Our study has several strengths, including the longitudinal approach, the lack of consent bias, and the large sample size, which allowed for sufficient power to detect a meaningful difference in B12 status between South Asian and European pregnant women. In addition, we performed a comprehensive assessment of B12 status using multiple direct and functional biomarkers over two time points during pregnancy. Yet, we acknowledge some limitations. As this study involved retrospective access to bio-banked serum samples, the sampled population may be biased. Records estimate that, depending on the age group, between 35·8 and 68·8% of pregnant women living in BC, Canada, chose to participate in the genetic screening programme in 2015( 44 ). Yet, the women participating in the programme might be older, have experienced previous pregnancy complications, or may be more anxious, educated or health conscious than the general population of pregnant women( Reference Metcalfe, Lix and Johnson 43 , Reference Goel, Glazier and Holzapfel 79 ). We conclude that although women were not actively recruited, the study may have a sampling bias and thus, may not be representative of the general population.
In summary, women of South Asian compared with European ethnicity living in Canada have a substantially lower B12 status in early pregnancy. Given that B12 is important for a healthy pregnancy and that South Asians are Canada’s largest ethnic minority, these results warrant future research on identifying predictors and potential health consequences of perinatal B12 inadequacy, especially in women of South Asian ethnicity.
Acknowledgements
The authors would like to thank the staff at the BC Prenatal Screening Laboratory for their support in sample collection; Matthew Saunders, Ori Nevares and Pablo Elizondo from UBC for their support in sample processing; and Arianne Albert from the Women’s Health Research Institute for statistical support.
This study was supported by the Canadian Institutes for Health Research/Canada Research Chair Program, and a grant of no charge materials from Abbott Laboratories. The funding sources had no influence on the design of the study, data analysis or writing of the manuscript.
T. H. S. and Y. L. designed the study and led the sample and data analysis; G. S. and H. D. V. provided input on study execution; G. S., A. M., S. I. B. and H. D. V. contributed to data interpretation. B. J. led the serum total B12 analyses. T. H. S. wrote the initial draft of the manuscript; and all authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










