Cellular DNA may be protected against oxidation by antioxidants, and oxidised DNA lesions are removed by several repair systems such as base excision repair, nucleotide excision repair and mismatch repair that have overlapping specificity and may interact or function as back-up systemsReference Dizdaroglu1. Low activity of DNA repair towards oxidised DNA may increase the risk of cancer by increasing the accumulation of errors in the genome. It has been reported that low repair activity towards oxidised DNA is associated with increased risk of cancer in case–control studies, although this could be due to reverse causalityReference Paz-Elizur, Krupsky, Blumenstein, Elinger, Schechtman and Livneh2, Reference Paz-Elizur, Ben-Yosef, Elinger, Vexler, Krupsky, Berrebi, Shani, Schechtman, Freedman and Livneh3. The assessment of DNA damage has been widely discussed and evaluated in dietary intervention studies, whereas assays for the assessment of DNA repair are few and data are limitedReference Møller4.
In principle there are two different approaches to measure DNA repair activity in cells. Lesions can be introduced in cellular DNA and the repair activity is assessed as the removal of lesions during a subsequent incubation period. The most popular type of repair activity measured by the single cell gel electrophoresis (comet) assay has been the measurement of rejoining of strand breaks after ex vivo exposure to clastogens such as H2O2 or ionising radiation. Although these assays for DNA repair activity are simple, they suffer from the drawback that the genotoxic exposure may damage the repair proteins and initiate the process of programmed cell death (apoptosis). Moreover, strand breaks are generated in cultured lymphocytes as a result of exposure to atmospheric O2 and they can also accumulate as a consequence of poor repair activity. The validity of repair assays based on the rejoining of strand breaks in antioxidant intervention studies has been questioned because they cannot distinguish between rejoining of strand breaks and ex vivo scavenging effects by antioxidantsReference Torbergsen and Collins5. These problems can be overcome by assays where extracts of cells are incubated with substrate DNA containing a defined number of lesions. Recently, a novel version of the comet assay was developed for the measurement of oxoguanine repair activity of cell extracts using substrate nuclei treated with the Ro19-8022 photosensitiser and white lightReference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6. This photosentiser generates 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and very few strand breaksReference Pflaum, Will and Epe7, Reference Will, Gocke, Eckert, Schulz, Pflaum, Mahler and Epe8. The repair activity is measured as the number of incisions that are generated during the incubation. This approach has been used in a few studies and indicates that dietary antioxidants can alter DNA repair activityReference Astley, Elliott, Archer and Southon9–Reference Tomasetti, Alleva and Collins11. The glycosylase enzymes incise at the sites of oxidised bases, leaving apurinic/apyrimidinic sites that are converted into breaks in the assay. The repair activity of Ro19-8022-damaged DNA is believed to mainly represent oxoguanine glycosylase 1 (OGG1)Reference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6, whereas nei-endonuclease VIII-like (for example, NEIL1) glycosylase and nucleotide excision repair enzymes probably have little contribution to the overall repair phenotype. The aim of the present study was to investigate if fruit and vegetable intake or antioxidant supplementation modulate the oxoguanine repair activity in mononuclear blood cells (MNBC) of human subjects. The reliability of the repair assay was assessed in liver homogenate tissues of wild-type and Ogg1 knockout mice.
Materials and methods
We investigated the DNA repair activity of cryopreserved MNBC from two antioxidant intervention studies that have been described in detail previouslyReference Møller, Vogel, Pedersen, Dragsted, Sandström and Loft12–Reference Dragsted, Pedersen and Hermetter14. The characteristics of the subjects enrolled in the two studies are outlined in Table 1. The level of oxidised purines was measured as formamidopyrimidine DNA glycosylase (FPG)-sensitive sites by the comet assay; these lesions are mainly represented by 8-oxodG and ring-opened purine lesions.
Table 1 Characteristics of subjects in the ‘six-a-day’ and vitamin C and E studies (Mean values and standard deviations)
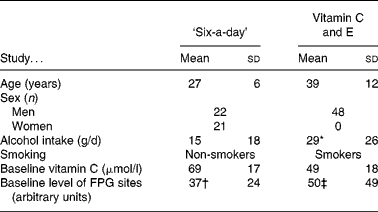
FPG, formamidopyrimidine DNA glycosylase.
* Assuming that one drink contains 16 g alcohol.
† Reported in Møller et al. (12) and Dragsted et al. (14).
‡ Reported in Møller et al. (13).
Characteristics of study 1 – ‘six-a-day’ study
The ‘six-a-day’ study was a parallel placebo-controlled intervention study in which forty-three subjects were randomised into three groups ingesting an energy-balanced basal diet free from fruits, vegetables and antioxidants, and supplemented with (1) 600 g fruits and vegetables, (2) tablets containing the corresponding amounts of vitamins and minerals, or (3) placebo tablets. The study is best characterised as a combined depletion and supplementation study. Blood and urine samples were collected at baseline (before) and at days 9, 16 and 24 of the supplementation. Post-intervention samples were obtained 4 weeks after the intervention had ceased. The constituents of the fruit and vegetable diet and content of the tablets have been described in detail previouslyReference Møller, Vogel, Pedersen, Dragsted, Sandström and Loft12, Reference Dragsted, Pedersen and Hermetter14. The samples analysed in the present study were obtained at baseline (pre-intervention), end-supplementation (day 24) and post-supplementation (day 53).
The subjects enrolled in the study had a high baseline plasma concentration of vitamin C. The concentration of plasma vitamin C decreased dramatically in the placebo group (the mean concentration of vitamin C was 21 μmol/l at the end of the supplementation). The profile of carotenoids was somewhat more complex; the plasma lycopene concentration only increased in the group that ingested 600 g fruits and vegetables/d, whereas the plasma β-carotene concentration increased the most in the group of subjects receiving tabletsReference Dragsted, Pedersen and Hermetter14.
Characteristics of study 2 – vitamin C and E study
The vitamin C and E study was designed as parallel, blinded, placebo-controlled investigation with forty-eight male smokers randomised into three groups as follows: (1) tablets with plain-release formulation of vitamin C (2 × 250 mg/d) and vitamin E (2 × 91 mg/d); (2) tablets with slow-release formulation of vitamin C (2 × 250 mg/d) and vitamin E (2 × 91 mg/d); (3) placebo. Whereas the content of vitamin C in the tablets is identical, ingestion of the tablets yields pharmacokinetically different variables due to the differences in release of vitamin C (for example, there is less fluctuation of the plasma concentration by ingestion of the tablets with slow-release formulation of vitamin C as compared with the plain-release tablets). On the contrary, the different formulations of the tablets have little pharmacokinetic effect on the plasma concentration of vitamin EReference Viscovish, Lykkesfeldt and Poulsen15. The subjects ingested tablets daily for 4 weeks. The samples analysed in the present study were obtained at baseline (pre-supplementation) and at the end of the supplementation (end-supplementation).
Measurement of DNA repair activity
The validation of the repair assay was assessed in liver extracts from wild-type and Ogg1 − / − mice. Afterwards, the DNA repair activity was determined in MNBC of the two intervention studies. Assessment of DNA repair incisions was analysed by the comet assay as described by Collins et al. Reference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6, with modifications as described below.
Preparation of cells with substrate nuclei
We used A549 lung epithelial cells as substrate nuclei (American Type Culture Collection, Manassas, VI, USA). These were washed twice with PBS before treatment. The A549 cells were treated with a 1 μm solution of the Ro19-8022 photosensitiser (a gift from Hoffmann-La Roche, Basel, Switzerland) dissolved in PBS, and irradiated for 4 min at 33 cm from two 500 W halogen lamps. The irradiation of the cell suspension was carried out on ice to avoid heating of the solution. After the treatment, cells were centrifuged at 700 g for 15 min to remove the photosensitiser, washed in RPMI-1640 medium (Gibco RBL, Grand Island, NY, USA) and centrifuged at 400 g for 15 min. The pellet was re-suspended in freezing medium (50 % fetal bovine serum (Gibco RBL), 40 % RPMI-1640 medium and 10 % dimethyl sulfoxide) to a concentration of 3 × 106 cells/ml and cryopreserved in small samples at − 80°C.
Preparation of mouse liver extract
Extracts of livers were obtained by placing the tissue into a stainless-steel cylinder and by use of a plunger forcing it through a sieve in one end of the cylinder (0·5 cm diameter, mesh size 0·4 mm), while the cylinder was submerged in 2 ml ice-cold buffer A (45 mm-HEPES, 0·4 m-KCl, 1 mm-EDTA, 0·1 mm-dithiothreitol, 10 % glycerol, adjusted with KOH to pH 7·8) according to the procedure described by Møller et al. Reference Møller, Wallin, Vogel, Autrup, Risom, Hald, Daneshvar, Dragsted, Poulsen and Loft16. Subsequently, the extracts were cryopreserved at − 80°C. The Ogg1 − / − mouse liver was a cryopreserved sample used in a previous studyReference Risom, Dybdahl, Møller, Wallin, Haun, Vogel, Klungland and Loft17. On the day of the experiment, samples of the cryopreserved samples were thawed and processed as described for MNBC.
Preparation of cell extract from human mononuclear blood cells
The same isolation procedure of MNBC was used in the ‘six-a-day’ and vitamin C and E studies. MNBC from venous blood were isolated in cell preparation tubes and cryopreserved in freezing medium (50 % fetal bovine serum, 40 % RPMI-1640 medium and 10 % dimethylsulfoxide). The cryopreserved cell samples were thawed and 5 ml of a 3-fold diluted extraction buffer A were added. The number of cells was ascertained and the samples centrifuged at 700 g for 5 min at 4°C. As much as possible of the supernatant fraction was removed. The pellet was re-suspended by vigorously tapping the tube and 20 μl buffer A was added for each 106 cells. The suspended cells were divided into 50 μl samples and frozen at − 80°C. This procedure of MNBC extract generation deviated from the original procedureReference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6, but we obtained similar incision activity in control experiments where we compared the two extract isolation procedures (results not shown).
In vitro repair incubation
Frozen samples of MNBC extracts were thawed and 12 μl 1 % Triton X-100 in buffer A were added. The lysate was subsequently centrifuged at 13 500 rpm for 5 min at 4°C to remove cell debris. The supernatant fraction was mixed with 4 volumes buffer B (40 mm-HEPES, 0·1 m-KCl, 0·5 mm-EDTA, bovine serum albumin (0·2 mg/ml), pH 8) and kept on ice until use. Samples of cells with substrate nuclei were thawed, mixed with low-melting-point gel (0·75 %; Sigma-Aldrich, Brøndby, Denmark) and applied on 85 × 100 mm GelBond® films (Cambrex, Medinova Scientific A/S, Hellerup, Denmark). Each GelBond consisted of eight 19 × 23 mm agarose gels. The GelBonds were immersed in lysis solution (2·5 m-NaCl, 0·1 mm-Na2EDTA, 10 mm-tri (hydroxymethyl)-aminomethane (Tris), 1 % Triton X-100, pH 10) for 1 h at 4°C. Then the GelBonds were washed three times (5 min each) in buffer B. Cell extract (60 μl) was added to each gel and incubated for 20 min at 37°C in a humid box. Control gels were incubated for 20 min with 60 μl of a control solution consisting of Triton X-100 and buffer B (without lymphocyte extract). The GelBonds were then placed in a horizontal electrophoresis tank and immersed in fresh solution (0·3 m-NaOH, 1 m-Na2EDTA) for 40 min, before electrophoresis at 25 V (0·83 V/cm) and 300 mA for 20 min. After neutralisation with 0·4 m-Tris-HCl (pH 7·5) cells were placed in 96 % ethanol for 1·5 h or overnight. The nuclei were visualised in an Olympus fluorescence microscope at 40 × magnification and two gels of 100 nuclei were scored as degrees of migration in classes 0–4 after staining with 50 μl YOYO-1 (Molecular Probes, Leiden, The Netherlands) in PBS buffer. The repair activity of the MNBC extract was determined as the difference in score (arbitrary units) between parallel gels incubated with extract and control solution.
Statistics
Differences in the baseline values of DNA repair phenotype of the two datasets were tested by ANOVA with unequal variance of groups because of lack of homogeneity of the variance (Levene's test). Effects of the intervention were tested by one-factor repeated-measurements ANOVA of two groups (placebo group v. active group). Since the data from the placebo groups were tested twice against the active groups, the statistical significance was accepted at P < 0·025 after Bonferroni correction for multiple tests. Analysis of normal distribution of residuals was tested by the Shapiro–Wilk W test. The relationship between DNA repair incision activity and FPG sites in baseline samples was analysed by linear regression with P < 0·05 as the significance level. The statistical analysis was carried out in STATISTICA version 5.5 for Windows (Statsoft, Inc., Tulsa, OK, USA).
Results
The DNA repair assay was implemented with several modifications compared with the original protocol, but we essentially observed similar time curves of the incision activity as reported previously, i.e. an initial linear increase of incisions followed by a plateauReference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6. We found that the most reliable incision activity was observed after 20 min incubations, based on considerations that the number of incisions should be as high as possible before the plateau is reached (results not shown). Fig. 1 outlines the results from experiments of dilutions of enzyme extract from the liver of mice. The protein concentration of the undiluted liver extract was 4 mg/ml, which was lower than that used for MNBC (about 20 mg/ml) because we assumed higher repair activity of the hepatocytes compared with MNBC. The 10-fold dilution of the wild-type liver extract reduced the incision activity to 43 (95 % CI 21, 65) %. The repair incision activity of the Ogg1 − / − mouse liver extract was very low, i.e. 17 (95 % CI 0, 39) % compared with the undiluted wild-type mouse liver sample, but it was not significantly lower than the 100-fold and 1000-fold diluted wild-type mouse liver extract samples.
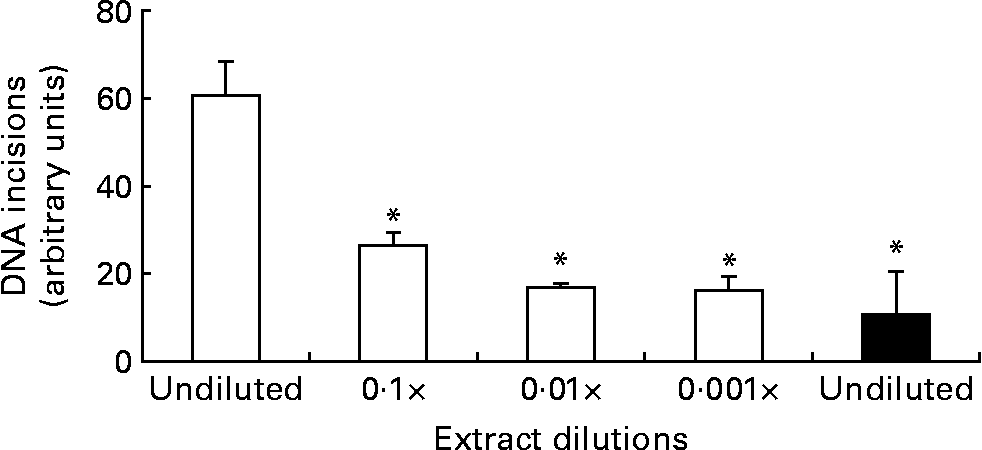
Fig. 1 DNA repair incisions of wild-type (□) and Ogg1 − / − (■) mouse liver extract. Values are means of three independent experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of the undiluted wild-type liver extract (P < 0·001; ANOVA).
The results of the baseline values in the two intervention studies are shown in Fig. 2. As can be seen, the distributions are quite different (P < 0·001; Levene's test). The values of the poorly nourished smokers in the vitamin C and E study are less scattered than the results from well-nourished subjects in the ‘six-a-day’ study, although there was difference in repair incisions between the placebo and slow-release groups at baseline (P < 0·05; one-factor ANOVA). The mean repair incision activity was higher for the subjects in the ‘six-a-day’ study (mean 86·1; 95 % CI 76·2, 99·9) than in the vitamin C and E study (mean 65·2; 95 % CI 60·4, 70·0; P < 0·001; ANOVA for groups with unequal variances). The residuals of this analysis did not deviate from a normal distribution (P = 0·12; Shapiro–Wilk W test), indicating little unexplained variation. The subjects in the vitamin C and E study were smokers, who had higher alcohol consumption, lower plasma vitamin C concentration, and higher levels of FPG sites in MNBC at baseline (Table 1). Although, at group level, there appeared to be an inverse relationship between the level of DNA repair incisions and FPG sites, there was not a statistically significant linear relationship between these biomarkers in the ‘six-a-day’ and vitamin C and E studies (P>0·05; linear regression analysis).

Fig. 2 Distributions of baseline DNA repair incision of mononuclear blood cell of subjects in the ‘six-a-day’ (■, –) and vitamin C and E (□, ‐ ‐) intervention studies. The data are repair incisions measured in arbitrary units.
Table 2 outlines the results of repair phenotype of MNBC extracts. The analysis of DNA repair phenotype was successful in forty-six samples of the vitamin C and E study, encompassing eighteen datasets in each of the active supplementation groups and ten subjects in the placebo group. In the ‘six-a-day’ study, samples from thirty-nine subjects were available and analysed, which included twelve, thirteen and fourteen subjects in the vitamins, fruit and vegetable, and placebo group, respectively. There was no significant variation in the DNA repair phenotype related to the intervention (P>0·025; ANOVA for repeated measurements). On the contrary, ingestion of slow-release vitamin C formulation for 4 weeks in heavy smokers was associated with a significantly increased repair capacity (P < 0·025; repeated-measurement ANOVA), whereas there was no difference in DNA repair incisions between subjects supplemented with tablets with plain-release formulation of vitamin C and placebo (P>0·025; repeated-measurement ANOVA). In Fig. 3, changes in DNA repair activity observed in the present study are combined with changes in FPG sites previously reportedReference Møller, Viscovich, Lykkesfeldt, Loft, Jensen and Poulsen13. The alteration of FPG sites is virtually a mirror image of the change in DNA repair.
Table 2 DNA repair incisions in mononuclear blood cell extracts of subjects in the ‘six-a-day’ and vitamin C and E studies* (Mean values with their standard errors)
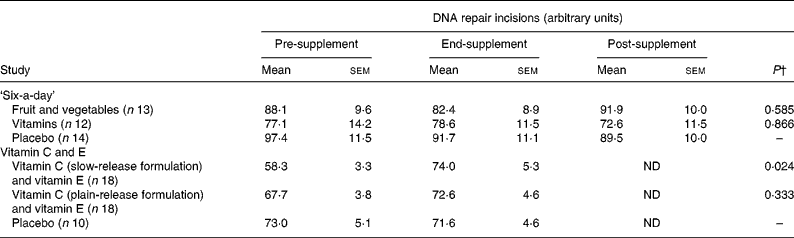
Pre-supplement, baseline; end-supplement, end of the supplementation period; post-supplement, 4 weeks after the end of the supplementation; ND, not determined.
* DNA repair incisions are obtained as the difference in score (arbitrary units) of substrate samples (i.e. A549 cells exposed to 1 μm-Ro19-8022 and white light) treated with mononuclear blood cell extract and buffer. The P values correspond to statistical analysis of repeated-measurement analysis that tests the difference in changes over time between the treatment and placebo groups.
† Effects of the intervention were tested by one-factor repeated-measurements ANOVA with P < 0·025 as the level of statistically significant effect.
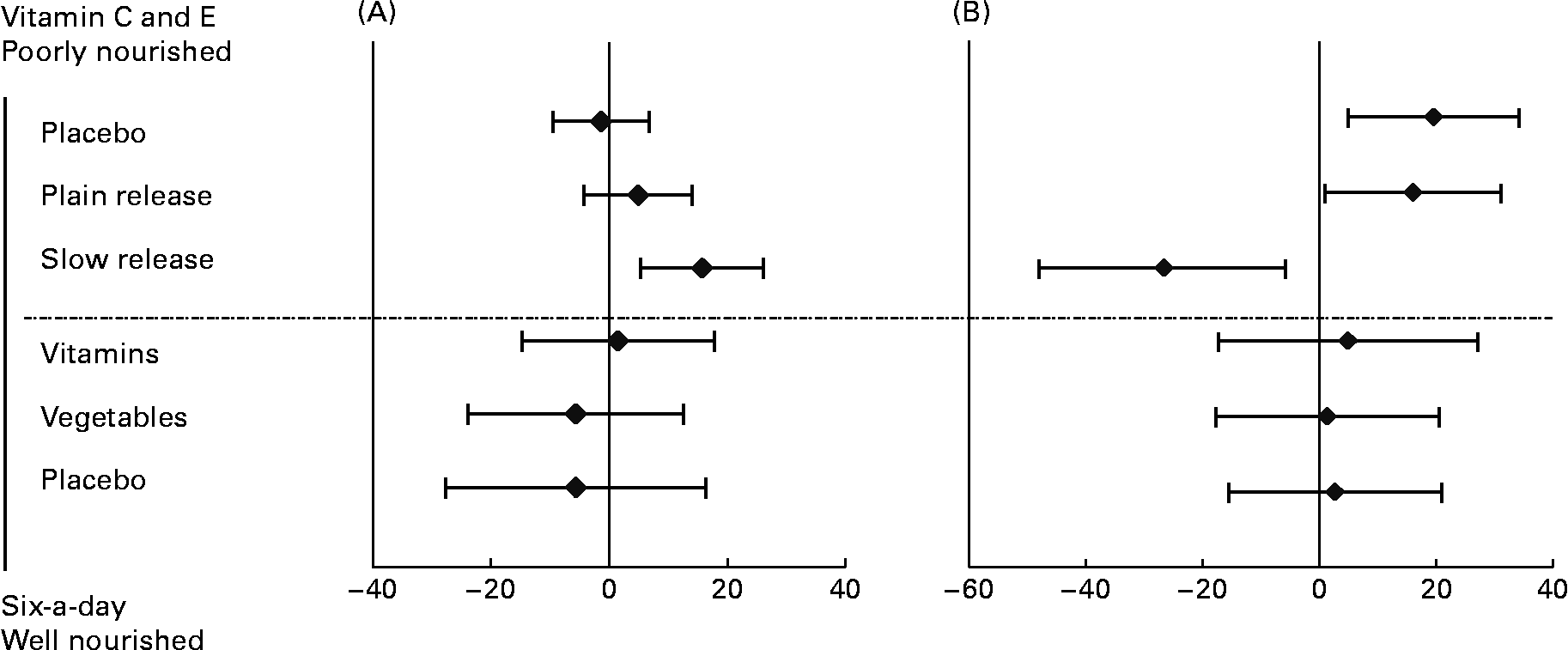
Fig. 3 Changes in repair activity (as difference of incisions in arbitrary units) (A) and formamidopyrimidine DNA glycosylase (FPG) sites (as difference in FPG sites in arbitrary units) (B) in mononuclear blood cells of subjects in the ‘six-a-day’ and vitamin C and E studies. In the vitamin C and E study subjects were supplemented with tablets containing 500 mg vitamin C/d as either a plain- or slow-release formulation together with 182 mg vitamin E/d. The subjects in the ‘six-a-day’ study received either 600 g fruits and vegetables/d or tablets containing the corresponding amounts of vitamins and minerals. Data are the differences between post- and pre-intervention. Values are means, with 95 % CI represented by horizontal bars.
Discussion
Oxidised DNA in cells is usually kept at a low steady-state level because the DNA repair system removes lesions from the DNA. However, during oxidative stress, the level of oxidised DNA in cells may increase because the DNA repair system cannot remove lesions with sufficient speed. Lower levels of oxidised DNA following supplementation with phytochemicals suggest that the bioactive constituents may increase the activity of the DNA repair system in addition to a direct scavenging effect of reactive oxygen species.
Methods for the assessment of DNA repair activity are scarce despite their relevance for understanding the cellular response to oxidative stress. The novel application of the comet assay for repair incision activity of cellular extracts is still relatively new and only a few groups have reported results from investigations with this method. Therefore we devoted some time to reassessing the initial assay validation carried out by Collins and co-workers, who reported that fibroblast cell cultures from Ogg1 − / − mice had very little incision activityReference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6. We found a dramatically reduced incision activity of the diluted wild-type extract, and 10-fold dilutions did not differ significantly from Ogg1 − / − liver extract, which had virtually no incision activity on Ro19-8022 substrate nucleoids. These results are in accordance with those reported by Collins et al. Reference Collins, Dusinska, Horváthová, Munro, Savio and Stetina6 who showed virtually no incisions by four-fold dilution of lymphocyte extracts.
Reliable assays for the measurement of repair of oxidised DNA bases by human MNBC cell extracts have become increasingly popular in recent years. However, investigations have mainly been carried out in small groups of subjects and high inter-individual variation can be difficult to separate from assay variation. In the present study we investigated the repair activity of MNBC extracts in two populations with different characteristics. This showed that at baseline there was not only a difference between subjects, but also differences between the groups of the population in the level of repair activity. Baseline differences in effect markers can be observed in parallel designs because of the unintentional heterogeneous randomisation of subjects. We do not believe that it affects the interpretation of the data because we have not selected participants with low incision activity that otherwise could lead to a regression towards the mean phenomenon. In addition, the data obtained in the vitamin C and E study are not unreasonably low; for example, we have previously found incision activities in the range of 50 arbitrary units in young and healthy subjectsReference Bräuner, Forchhammer, Møller, Simonsen, Glasius, Wåhlin, Raaschou-Nielsen and Loft18. However, problems with baseline differences in biomarkers can be alleviated by the use of cross-over designs, but this type of design suffers from other problems such as carry-over effects, and systematic variation over time can be particularly troublesome. The overall mean of baseline repair activity for both datasets was 76·7 (sd 29·6) with a corresponding CV of 39 %, but the data are not normally distributed. This is similar to the CV (34 %) reported in a study on 375 subjectsReference Dusinska, Dzupinkova, Wsolova, Harrington and Collins19, whereas another study among 244 subjects reported a CV in the range of 100 %Reference Vodicka, Stetina and Polakova20. The repair incision activity in these subjects was reported to correlate weakly and positively with ageReference Dusinska, Dzupinkova, Wsolova, Harrington and Collins19 or not being age dependentReference Vodicka, Stetina and Polakova20. Sex and smoking habit were not reported to affect DNA repair activity towards oxidised bases, whereas polymorphisms in the OGG1 and XPA genes affected the repair incision activityReference Dusinska, Dzupinkova, Wsolova, Harrington and Collins19, Reference Vodicka, Stetina and Polakova20. This indicates that that age, sex and smoking are not strong determinants for the repair incision activity and the differences we have observed between the subjects in the ‘six-a-day’ and vitamin C and E studies could be due to other lifestyle and genetic variables. It should be emphasised that the repair activity of the two datasets were analysed at the same time, and the difference cannot be attributed to variation in the analysis over time. The ‘six-a-day’ samples had been stored for a longer time (6 years) than the samples from the vitamin C and E study (4 years). A recent study did not show decreased repair activity, determined as cleavage of 8-oxodG-containing oligonucleotides, in samples after storage for 3 yearsReference Paz-Elizur, Elinger, Leitner-Dagan, Blumenstein, Krupsky, Berrebi, Schechtman and Livneh21. Clearly, further studies are warranted that assess the stability of repair activity during storage.
The effect of phytochemical supplementation on DNA damage has been investigated in more than 100 studies, whereas few studies on repair activity are availableReference Møller4. Increased DNA repair activity was observed after supplementation with kiwi fruits, and this was accompanied by decreased levels of oxidised DNA in MNBCReference Collins, Harrington, Drew and Melvin10. Using a different assay for repair activity, cell extract-associated incision activity of plasmid DNA treated with methylene blue and visible light, it was indicated that ingestion of cooked carrots increased repair activity, whereas ingestion of mixed carotene tablets and tinned mandarin oranges had no effect on DNA repair activityReference Astley, Elliott, Archer and Southon9. Increased repair incisions of Ro19-8022-induced DNA damage were also observed following a 1-week supplementation with coenzyme Q10, whereas the levels of FPG sites in MNBC did not changeReference Tomasetti, Alleva and Collins11. Our observation that increased incision repair activity was induced by vitamin C supplementation corroborates well with these results, and indicates that it is possible to increase repair activity toward oxidised DNA in MNBC by supplementation with phytochemicals and antioxidants. It is all the more interesting that it was not possible to alter the repair incision activity in MNBC of the well-nourished subjects in the ‘six-a-day’ study. Measurements of the OGG1 mRNA levels in whole blood of the subjects were not altered because of the treatment in the present study, and the vitamin C and E supplementation study, although period effects were observed in both studiesReference Møller, Vogel, Pedersen, Dragsted, Sandström and Loft12, Reference Møller, Viscovich, Lykkesfeldt, Loft, Jensen and Poulsen13, Reference Vogel, Møller, Dragsted, Loft, Pedersen and Sandström22. However, the data on OGG1 mRNA levels are not directly comparable with the measurements of DNA repair activity because the latter was investigated in MNBC. The unaltered incision repair activity of the subjects in the ‘six-a-day’ study might be explained by the short duration of the depletion period in these well-nourished subjects. The lifespan of lymphocytes, which is the major fraction of MNBC, is usually considered to be long (probably years), although it is likely to depend on the subtype of lymphocytes. The depletion of phytochemicals in the ‘six-a-day’ study was only 24 d, which is a short time compared with the lifespan of lymphocytes. Thus, it is reasonable to expect that a large fraction of the cells in the blood remained unaltered after 24 d. A similar lack of effect was observed for antioxidant enzyme activity (including catalase, superoxide dismutase and glutathione reductase) in erythrocytes, which only have a lifespan of 120 d, but glutathione peroxidase in the erythrocytes was significantly increased by the fruit and vegetable intervention, indicating that changes in some biomarkers over a shorter time period may take place even in cell populations which have long half-lives even without de novo protein synthesisReference Dragsted, Pedersen and Hermetter14. It had been shown that the ex vivo sensitivity to oxidatively induced DNA damage increased in subjects with reduced antioxidant levels at baseline associated with a controlled run-in period with a diet poor in antioxidantsReference Lean, Noroozi, Kelly, Burns, Talwar, Sattar and Crozier23, Reference Riso, Visioli, Erba, Testolin and Porrini24. Taken together, the data suggest that the subjects in the ‘six-a-day’ study might not have experienced significant oxidative stress following the 24 d depletion of antioxidants. Thus, the most important interpretation of these observations is that swift alterations in DNA repair enzyme activity can be observed by supplementation of antioxidants in poorly nourished subjects, whereas severe depletion of antioxidants for long periods of time might be required to observe alterations in well-nourished subjects.
In conclusion, determinants of the oxoguanine glycosylase repair phenotype encompass dietary habits, lifestyle factors and genetic differences. Subjects with poor habitual antioxidant intake may benefit from supplementation with concomitant reduction of the level of oxidised DNA in cells.
Acknowledgements
The technical assistance from Claus Nielsen and Gitte Friis is gratefully acknowledged. The present study was partly supported by ECNIS (Environmental Cancer Risk, Nutrition and Individual Susceptibility), a network of excellence operating within the European Union 6th Framework Program, Priority 5, ‘Food Quality and Safety’ (contract no. 513943) and the Danish Research Councils.









