Article contents
Effects of surface properties of red mud on interactions with Escherichia coli
Published online by Cambridge University Press: 16 May 2013
Abstract
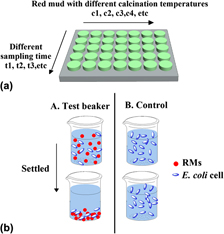
Adsorption of Escherichia coli (E. coli) cells on red mud (RM) is important in the interactions between RM and bacteria. The objective of this work is to study adsorption of E. coli onto RM and to determine its influence in relation to the surface properties of RM. The effects of different calcination temperatures on the surface properties of red mud were investigated by thermogravimetric analysis, x-ray diffraction, scanning electron microscopy, Brunauer, Emmett, Teller (surface measurement)/N2 adsorption method, and zeta potential analysis. A higher adsorption capacity was observed from RM calcinated at 700 °C (RM700) due to larger pores formed on the surface of RM. The correlation between the adsorption efficacy and surface properties of RM is discussed and the extended Derjaguin-Landau-Verwey-Overbeek theory suggests that when the adsorption reaches equilibrium, the increased adsorption of E. coli onto RM is due to the smaller energy barrier between E. coli and RM700 as compared with that between E. coli and raw RM (RM0).
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 17: Focus Issue: Advances in the Synthesis, Characterization, and Properties of Bulk Porous Materials , 14 September 2013 , pp. 2332 - 2338
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 3
- Cited by




