Subarachnoid hemorrhage (SAH) following ruptured intracranial aneurysm is often complicated by shunt-dependent hydrocephalus (SDHCP), clinically significant vasospasm, and delayed ischemic events. Intraoperative maneuvers to lower these complications, such as aggressive clot evacuation,Reference Hosoda, Fujita, Kawaguchi, Shose, Hamano and Iwakura 1 , Reference Mura, Rojas-Zalazar, Ruíz, Vintimilla and Marengo 2 blood vessel manipulation, and communication of ventricles to the cisternal space,Reference Akyuz and Tuncer 3 - Reference Komotar, Olivi, Rigamonti and Tamargo 6 have been advocated as an adjunct to aneurysm surgery. Microsurgical fenestration of lamina terminalis (FLT) and/or Liliequist membrane (LM) during aneurysm clipping, first described by Dandy and popularized by Yasargil to achieve brain relaxation,Reference de Divitiis, Angileri, d’Avella, Tschabitscher and Tomasello 7 , Reference Dandy 8 has been described to reduce the incidence of clinically relevant vasospasm and SDHCP. By providing a continuous cisternal lavage, ventriculocisternostomy is thought to improve cerebrospinal fluid (CSF) dynamics and prevent subarachnoid blood from causing arachnoid fibrosis and vascular inflammation.Reference Andaluz and Zuccarello 4 , Reference Auer and Mokry 9 - Reference Komotar, Hahn and Kim 11
We sought to study the role these intraoperative maneuvers during aneurysm surgery. Here, we show that the incidence of clinically relevant vasospasm is significantly lower when these intraoperative maneuvers are used during microsurgical clipping. This difference is more pronounced with higher Fisher grade (FG) SAH.
METHODS
Selection Criteria
Patients were identified retrospectively under an approved institutional review board protocol. The study population included patients aged 18 years or older (1) with acute SAH resulting from a ruptured anterior circulation aneurysm who presented at our service between January 2008 to December 2013; (2) who underwent pterional craniotomy, which allows surgical access to the lamina terminalis and LM during aneurysm clipping; and (3) in whom presence of intracranial aneurysms were confirmed radiographically by computed tomography (CT) angiogram (CTA) or conventional four-vessel diagnostic cerebral angiogram (DSA). All surgeries were performed by two surgeons (A and B), with (group A) or without (group B) meticulous clot evacuation, establishing CSF dynamics by creating alternate routes (i.e. FLT and FLM [Fenestration of Lamina Terminalis]) and adventitial dissection of major vessels using papaverine. All postoperative care was provided in the Neuroscience Intensive Care Unit using uniform institutional protocols for management of aneurysmal SAH, thus minimizing patient–patient variation in postoperative care.
KEY DEFINITIONS
The presence of SAH was confirmed either by CT imaging or by lumbar puncture demonstrating xanthochromia and increased red cells in the CSF of patients with clinical suspicion of a ruptured intracranial aneurysm. Vasospasm was characterized on days 3 through 14 post-SAH based on (1) angiographic evidence of vascular narrowing in the moderate, moderate to severe, and severe category as read by neuroradiologists; (2) whether vasospasm required intervention (such as verapamil infusion or balloon angioplasty) during angiography; (3) development of a delayed ischemic neurological deficit (DIND) (i.e. a delayed neurological deficit [3-14 days after SAH]) that is not explained by other causes (such as hydrocephalus, medications, seizures, etc); and (4) presence of new hypodense areas on postsurgery CT imaging during the susceptibility period (i.e. postsurgery days 3-14). All patients had a head CT scan on postoperative day (POD) 1 to evaluate immediate postsurgical strokes (e.g. perforator infarcts) and edema. Thus, only new areas of hypodensity on PODs 3 through 14 were considered new strokes. Mortality and morbidity outcome in the two groups was determined by Glasgow Outcome Scale (GOS) and modified Rankin scale (mRS).
Statistical Analysis
Student’s two-sample t test and Wilcoxon rank-sum test were used for univariate comparisons between group means and medians. Chi-square and Fisher exact tests were used for univariate comparisons between categorical variables. Multivariable logistic regression was performed for each of the following independent, dichotomous outcomes: angiographic vasospasm, DIND, and occurrence of new stroke. Stepwise and backward variable selection methods were used to select independent predictors for each of these outcomes. The following predictors were included in the initial regressions: age, sex, Hunt and Hess (HH) score (≤3 vs >3), FG (≤3 vs 4), initial Glasgow Coma Scale (GCS; ≤9 vs >9), as well as all second-order interactions. Adjusted odds ratios and their 95% confidence intervals were obtained for these outcomes. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Population Characteristics
Of the 562 patients screened (including those with traumatic SAH), 159 satisfied the inclusion criteria (Table 1). Of those, 114 (71.6%) were in group A and 45 (28.3%) in Group g with a mean age (standard deviation) of 55.4 (12.3) years and follow-up of 184 days. Patients in group A were slightly older than those in group B: 56.7 (12.4) versus 52.2 (11.7) years, respectively (p=0.04). More than 60% of patients presented with an HH score of 3 or more and FG of 4. Between the two groups, length of intensive care unit and total hospital stay were similar (p=0.55 and 0.37, respectively). More than 45% of aneurysms were in the anterior communicating artery region, 28% in the middle cerebral artery, and 17% in the posterior communicating artery, with similar distribution between the two groups. Almost two-thirds of all ruptured aneurysms were <7 mm in size, whereas 20% were 7 to 13 mm and <10% were >13 mm.
Table 1 Clinical and radiographic characteristics
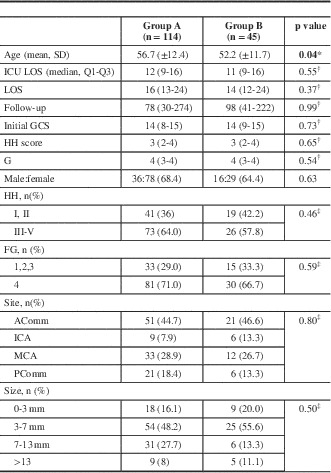
* p value obtained from two-sample t test for means.
† p value obtained from the exact Wilcoxon two-sample test.
‡ p value obtained from the chi-square test for independence.
Values in bold indicate statistically significant differences.Acomm=anterior communicating artery; FG=Fisher grade; GSC=Glasgow coma score; HH=Hunt and Hess; ICA=internal carotid artery; ICU=intensive care unit; LOS=length of stay; MCA=middle cerebral artery; Pcomm=posterior communicating artery.
Vasospasm
Based on radiologist’s interpretation of pretreatment angiogram (usually a CTA), the incidence of pretreatment vasospasm was similar between the two groups (p=0.52). All patients had a DSA between days 3 and 10 (median, 7 days) after aneurysm treatment. Overall, 36% of patients developed moderate or higher degrees of angiographic vasospasm (Table 2). There was a nonsignificant trend toward less radiographic vasospasm ipsilateral to the ruptured aneurysm (30.7% in group A and 46.7% in group B, p=0.06) and in all vessels (31.6% vs 47.7%, p=0.07) between the two groups. This difference reached statistical significance in the cohort of patients that presented with SAH with intraventricular hemorrhage (i.e. FG 4; 35.8% in aggressive vs 56.7% in standard surgical groups, p=0.05, ipsilateral vasospasm) (Table 2).
Table 2 Radiographic vasospasm
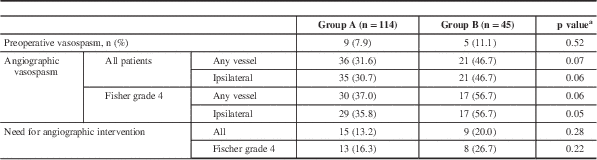
a Chi-square test for independence.
There was a statistically significant difference between the occurrences of DIND within the two groups (18.4% vs 37.8%, p=0.01). This effect was maintained in patients with FG 4 SAH (p=0.04). There was no difference in the need for angiographic interventions, such as balloon angioplasty or verapamil administration, to relieve vasospasm during surveillance DSA (Table 3).
Table 3 Clinical vasospasm

* p value obtained from the chi-square test for independence.
† p value obtained from Fisher’s exact test.
DIND=delayed ischemic neurological deficit; FG=Fisher grade.
The overall incidence of new strokes was 28.5%. In patients with moderate or higher degree of vasospasm, there were 30% fewer strokes in group A than in group B (46.4% vs 76.5%, p=0.06). In the same group of patients with FG 4 SAH, however, the difference was >50% (30% vs 64.7%, p=0.02) (Table 3).
Using multivariable logistic regression, we identified independent predictors for the occurrence of angiographic vasospasm, DIND, and new strokes. We found that as age increased, the odds of angiographic vasospasm decreased by 3% per year (95% confidence interval [CI]: 1%-5%). Subjects with HH scores ≥3 were 3.97 (95% CI: 1.96-8.01) times more likely than subjects with scores <3 to experience any vasospasm. Significant factors associated with DIND were HH score, with scores ≥3 increasing the odds of DIND by 2.82 (95% CI: 1.24-6.39), and an interaction between age and the FG. In patients with FG <4, the odds of DIND decreased by 6% per year (95% CI: 0.1-12). In subjects with FG 4 SAH, the odds of DIND did not change with increasing age. Significant factors associated with occurrence of new strokes on postintervention days 3 through 14 CT scans were HH score and the interaction between GCS and sex. Patients with HH scores ≥3 were 3.37 (95% CI: 1.48-7.70) times more likely than subjects with HH scores <3 to experience a new stroke. Males with initial GSC scores ≥9 were 88% (95% CI: 44-98) less likely than those with scores <9 to experience a new stroke. For female patients, the odds of new stroke did not change with GCS score. Both groups of patients improved on median GOS and mRS between discharge and follow-up, with no difference between the two groups (Table 4).
Table 4 Clinical outcome
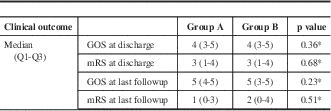
* p value obtained from the exact Wilcoxon two-sample test.
GOS=Glasgow Outcome Scale; mRS=modified Rankin Scale.
DISCUSSION
Incidence of Clinical Vasospasm
The incidence of vasospasm after SAH is 70%,Reference Adams, Kassell, Torner and Haley 12 with up to 40% of patients developing DIND.Reference Adams, Kassell, Torner and Haley 12 - Reference de Oliveira, Beck, Ulrich, Rathert, Raabe and Seifert 15 Hohlrieder et al reported the highest degree of vasospasm (82.4%) in patients with HH score of 4.Reference Hohlrieder, Spiegel and Hinterhoelzl 14 A pooled estimate of symptomatic vasospasm reported in six studies showed no difference between coiled or clipped groups (32.3% surgical vs 25.1% endovascular).Reference de Oliveira, Beck, Ulrich, Rathert, Raabe and Seifert 15 Andaluz et al reported that in patients undergoing clipping of anterior communicating artery aneurysms, clinical vasospasm developed in 29.6% of patients who underwent FLT compared with 54.7% of patients who did not.Reference Andaluz and Zuccarello 4 However, Komotar, in 2009, reported no benefit of FLT in development of clinical vasospasm.Reference Komotar, Hahn and Kim 16
In the present study, 35% of the patients developed moderate or higher degrees of angiographic vasospasm with a 23.9% incidence of clinical vasospasm (DIND). This is slightly lower than the pooled estimate of 32.3% reported in a recent meta-analysis.Reference de Oliveira, Beck, Ulrich, Rathert, Raabe and Seifert 15 This could partly be due to variability in how vasospasm is defined in literature. Compared with standard surgery, patients in group A had a 20% lower incidence of DIND (p=0.01), with a 14% reduction (p=0.06) in ipsilateral radiographic vasospasm. We further analyzed all patients with FG 4 SAH (greatest blood burden). We found that, in this group, 19 of 81 patients (23.8%) in group A and 13 of 30 (43.3%) in group B developed DIND. This nearly 20% reduction in DIND was statistically significant (p=0.04).
Thus, our data suggest potential benefit of an aggressive surgical approach based on a reduction of DIND by 20% in patients surgically treated for ruptured anterior circulation aneurysms.
Development of New Strokes
The incidence of new infarcts after an SAH is reported between 20% and 60%.Reference Hohlrieder, Spiegel and Hinterhoelzl 14 , Reference Petruk, West and Mohr 17 - Reference Kassell, Torner, Jane, Haley and Adams 22 In the nimodipine trial,Reference Petruk, West and Mohr 17 the incidence of new infarcts decreased from 53.3% to 42.4%. Gruber et al reported a higher incidence in endovascular group of 37.7% and 21.6% in the surgical group while qualifying that this difference was from a skewed FG 4 infarction in endovascular group.Reference Gruber, Ungersböck and Reinprecht 19 A recent meta-analysis reported pooled estimates of 16.5% new strokes in surgical groups and 22.0% in endovascular groups.Reference de Oliveira, Beck, Ulrich, Rathert, Raabe and Seifert 15
In our study, the overall incidence of new strokes was ~29%. The only group with significant differences in new stroke was the FG 4 group, where the percentage of patients with new stroke in group A was half that of group B (30% and 64%, respectively). It is, however, to be noted that because of the small number of patients in this subgroup, the results warrant further exploration.
Overall, this represents a 20% reduction in the incidence of clinically significant vasospasm and a 50% reduction in the development of new strokes in patients with high-grade aneurysm rupture (i.e. FG 4 with angiographic vasospasm), with the data reaching statistical significance.
Limitations
In addition to the retrospective nature of this single-institution study, a relatively small number of patients in our study preclude definitive conclusions. Differences in surgical experience of the two primary surgeons, in addition to surgical technique, could potentially have influenced our results. Moreover, surgeon B performed both open and endovascular procedures, lending the study to inherent selection bias.
Although group A included a number of intraoperative maneuvers including FLT and LM, use of papaverine in adventitial dissection of larger blood vessels, and aggressive clot removal, we cannot conclude which of these was responsible for the effects observed in our study. Additionally, patients in group A tended to be slightly older than those in group B; age is a confounding factor on development of angiographic vasospasm. However, the odds of DIND with increasing age were the same in subjects with FG 4. Despite reduction in clinically significant vasospasm (DIND and strokes) in the aggressive surgical group, the overall clinical outcome as assessed by GOS and mRS at discharge and last follow-up did not show a difference between the various groups. It has been suggested that the use of a gross outcome scale such as GOS may overlook subtle neuropsychological deficits that could be detected in patients with a “good” neurological outcome.Reference Berry, Jones, West and Brown 23 - Reference Ogden, Mee and Henning 25 The retrospective nature of the current study precluded detailed assessment of potential long-term differences in cognition, learning, and memory.
Surgical Treatment in the Era of Endovascular Aneurysm Treatment
Though there will undoubtedly be a role for surgical intervention for treatment of certain ruptured aneurysms, the safety and efficacy of endovascular techniques is continually improving. Overall outcomes have been shown to be improved by endovascular treatment compared with surgical treatment in two large randomized trials.Reference McDougall, Spetzler and Zabramski 26 , Reference Molyneux, Kerr and Stratton 27 It is therefore incumbent on surgeons to explore techniques that may improve outcomes in patients requiring surgery with regard to vasospasm and SDHCP. Having direct access to the region of concentrated subarachnoid blood and CSF cisterns seems to offer a theoretical opportunity to intervene to improve outcomes. Preliminary observations of additional “aggressive treatments” have shown some early promising results. Cisternal drainage has been shown to increase clot clearance rate.Reference Shirao, Yoneda and Ishihara 28 Techniques of cisternal lavage with head shaking has also shown promise in reducing stroke and vasospasm.Reference Shirao, Yoneda and Ishihara 28 Our data support the observation that surgical manipulation may have some beneficial effect. It will be incumbent on surgeons to examine in well-designed prospective trials which intraoperative maneuvers, available only to surgeons, either alone or in combination will reduce the delayed complications that too commonly occur following aneurysmal SAH.
CONCLUSION
We believe that aggressive clot removal and intraoperative papaverine-guided adventitial dissection during aneurysm surgery results in lower incidence of DIND and new strokes. This effect is most pronounced in patients with FG 4 SAH. Because of inherent limitations of this study, definite conclusions cannot be made and would involve further studies. Moreover, the relative contribution of individual components comprising “aggressive approach” on a lower incidence of DIND is unclear at this time.
Acknowledgments
Statistical support for this study was supported by National Institutes of Health grant no. 1UL1RR031977-01 (to MOC).
Disclosures
The authors have nothing to disclose.








