Introduction
Feed cost contributes up to 60–70% of the total production cost in pig farming. Energy and protein are the major cost components in the feed, and phosphorus (P) often accounts for the third most expensive component in pig diets. Due to limited natural resources, inorganic phosphate price has increased drastically in the last 10 years (von Horn and Sartorius, Reference von Horn and Sartorius2009). Organic phosphorus sources in plant based ingredients are mainly present in the form of phytate, which has limited availability to mono-gastric animals (Woyengo and Nyachoti, Reference Woyengo and Nyachoti2013). To overcome this, phytase enzyme is commonly used at an inclusion level of 500 FTU/kg in grower/finisher pig diets to breakdown phytate and increase phytate P digestibility. In practice, phytase is commonly used in pig feed as a partial replacement of inorganic phosphorus (iP) from monocalcium phosphate (MCP) or dicalcium phosphate (DCP). A total replacement of inorganic phosphorus by phytase will further reduce feed cost and allow further reduction of P excretion to the environment.
Recent studies showed that using phytase produced from the Buttiauxella organism at higher dose (e.g. 1000 FTU/kg) continued to increase the digestibility of P and amino acids (Adedokun et al. Reference Adedokun, Owusu-Asiedu, Ragland, Plumstead and Adeola2015), indicating both phosphoric and extra-phosphoric effects. Additionally, Remus et al. (Reference Remus, Dersjant-Li, Plumstead and Awati2015) observed that increasing Buttiauxella phytase dose from 500 to 1000 FTU/kg further improved production performance of growing pigs fed corn and soybean meal based diets.
The efficacy of phytase is influenced by a number of factors, including the choice of dietary main grain. Liu et al. (Reference Liu, Cadogan, Péronc, Truong and Selle2014) observed that addition of Buttiauxella phytase at dose of 1000 FTU/kg improved weight gain, feed intake and feed efficiency in corn, wheat or sorghum based diets in broilers. However, phytase addition to corn-based diets generated more pronounced responses in weight gain and feed conversion ratio (kg feed/kg BW gain, FCR) compared to sorghum or wheat based pelleted diets.
The diets used in these trials were relatively simple, while it is a common practice in Europe to formulate pig diets using mixed grain types (e.g. with wheat, barley, triticale or rye). To date, limited information is available on the effect of increasing phytase dose up to 1000 FTU/kg on production performance in grower/finisher pigs fed European type, multi-grain based complex diets. Therefore, the objective of the present study was to determine the effect of increasing Buttiauxella phytase dose up to 1000 FTU/kg on production performance in growing/finishing pigs fed wheat, corn, barley and soybean meal based diet without supplemental inorganic phosphorus.
Materials and methods
Animals and experimental facility
The study was conducted at the research facility of the University of Applied Sciences Bingen, Germany and was approved by the university's animal welfare committee. Pigs (cross-bred Topigs x Pietrain) were housed in a temperature controlled environment within the fattening barn on continuous solid floors bedded with straw, where 100 pens were used containing one pig per pen. During the trial period the temperature was maintained at 22°C. The pigs were individually ear-tagged and weighed after arriving at the research facility. After weighing, pigs were immediately assigned randomly to pens and allocated to one of the five experimental diets while the body weight (BW) was balanced across treatments and pens (equal average BW and standard derivation per pen). The trial design provided 20 replicates (individually housed pigs) per treatment and pigs (mean initial BW 30.8 ± 1.6 kg, 10 weeks of age) were equally distributed by gender (50 barrows and 50 gilts) and housed in two identical adjoining barns for a total trial period of 93 days.
Dietary treatments
Five dietary treatments were used on the trial, including a positive control (PC) formulated to meet the nutrient requirement of the pigs (GfE, 2006) supplemented with inorganic P from monocalcium phosphate (MCP), a negative control (NC) without inorganic P and with reduced Ca (−0.12%) and metabolisable energy (ME) content (−0.14 MJ ME/kg) or NC supplemented with a phytase at 250, 500 or 1000 FTU/kg respectively. The phytase was a microbial 6-phytase from Buttiauxella sp. expressed in Trichoderma reesei (Axtra® PHY, Danisco Animal Nutrition, DuPont Industrial Biosciences, Marlborough, UK). Phytase activities are defined as FTU and measured as the amount of phytase that liberates 1 mmol of P per minute from 0.0051 mol/l sodium phytate at pH 5.5 and at 37°C (AOAC, 2000).
The diet composition, calculated and analysed nutrients are shown in Table 1. Diets were based on wheat, barley, corn and soybean meal, formulated without enzymes (other than phytase) and did not contain antibiotics or any other growth promoters. All diets were formulated to not exceed EU maximum permitted concentrations for trace minerals or vitamin. Test diets were prepared in mash form, and, along with water, were supplied ad libitum throughout the trial period. Diets were provided in two feeding phases of 30–85 kg BW for grower phase and 85 −125 kg BW for finisher phase.
Table 1. Diet formulations, calculated and analysed nutrients (as fed)
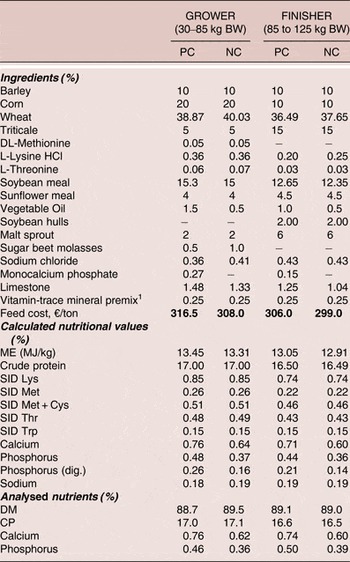
1Vitamin-mineral premix provided per kg of feed:
12,500 IU vitamin |A; 1,500 IU vitamin D3; 125 mg vitamin E; 200 mcg biotin; 1.0 mg folic acid; 3.5 mg vitamin B1; 7.0 mg vitamin B2; 6.0 mg vitamin B6; 50 mcg vitamin B12; 35 mg nicotine acid; 20 mg Ca-Pantothenate; 345 mg choline chloride; 300 mg choline; 50 mg Fe (sulphate); 12.5 mg Cu (sulfate); 40 mg Mn (oxide); 60 mg Zn (oxide); 1 mg J (Ca-jodate); 0.15 mg Co (carbonate); 0.35 mg Se (Na-selenite)
Sampling and measurements
From each feed batch, pooled samples were taken for phytase activity analysis at DuPont Nutrition Biosciences ApS, Brabrand, Denmark (Yu et al., Reference Yu, Cowieson, Gilbert, Plumstead and Dalsgaard2012; Reference Yu, Kvidtgaard, Isaksen and Dalsgaard2014) and for proximate analysis at R&D Centre laboratory Bingen. Crude protein, dry matter, Ca and P were analysed according to the methods of VDLUFA (Naumann and Bassler, Reference Naumann and Bassler1976; VDLUFA-Methodenbuch, 1993). Results of analysed phytase activity of the test diets are shown in Table 2.
Table 2. Analysed phytase activity (FTU/kg) in the diets

The body weight of each pig was recorded at the start of the trial, when feed phase changed (at 85 kg BW) and at the end of the trial (125 kg BW). Uneaten feed from each pen was weighed in order to record actual daily feed intake of the pigs. Average daily gain (ADG g/day), average daily feed intake (ADFI g/day), and feed conversion ratio (FCR kg feed/kg weight gain) were calculated. On day 70 back fat thickness was measured (ultrasonic P2-method) by trained personnel for all individual pigs. At the end of the experimental period (target BW 125 kg BW) all pigs were weighed, slaughtered at a commercial facility using CO2 gas and carcass characteristics were measured with Fat-O-Meater (FOM) technique. The following parameters were measured: slaughter weight (kg), lean meat percentage, muscle depth, back-fat thickness (Hennessy GP4), and carcass yield. Back fat thickness and muscle depth were measured longitudinally 6 cm from the mid-line between third/second last rib.
Data analysis
Each pig was considered an experimental unit and replicate in the trial. The effects of dietary treatment, gender and any interactions were examined in the Fit Model platform of JMP 11 (SAS Institute Inc., USA). Means were separated using Tukey's HSD, and homoscedasticity was tested using the Levene-Test model. Linear responses were analysed with increased phytase dose from 0 (NC) to 1000 FTU/kg. Significance was determined using 95% confidence limits (P < 0.05).
Results
Performance
Productive performance results are presented in Table 3. No treatment and gender interaction was found for any of the parameters measured. Overall mortality was very low and did not differ between treatments.
Table 3. Effect of increasing phytase dose on production performance in grower/finisher pigs1

1 Each value represents the mean of 20 individually-housed pigs
2 P phytase linear effect was analysed with dose increase from 0 (NC) to 1000 FTU/kg.
a,b : different superscript in a column indicates significant difference at P < 0.05
During the grower phase, increasing phytase dose from 0 (NC) to 1000 FTU resulted in a linear increase in ADG and BW, regardless of gender of the pigs. Pigs fed the NC diet had lowest ADG, NC + 500 FTU and NC + 1000 FTU/kg phytase improved (P < 0.05) ADG compared to NC, while PC and 250 FTU/kg phytase treatments showed intermediate response on ADG. Phytase supplementation at 250, 500 and 1000 FTU/kg to NC diet improved ADG by 3.5, 7.2 and 8.1% respectively compared to NC and by 0.8, 4.5 and 5.3% respectively compared to the PC. ADFI and FCR were not significantly influenced by dietary treatments, however, phytase addition numerically reduced FCR compared to both the NC and PC. Phytase at 250, 500 and 1000 FTU/kg reduced FCR by 2.0, 2.8, and 3.7% respectively compared to the NC, and by 1.2, 2.0 and 2.9% respectively compared to the PC. During the grower phase, gilts had lower ADFI, ADG and FCR (P < 0.05) compared to barrows.
Pig performance in the finisher phase and overall period did not differ between dietary treatments, although pigs fed the NC diet had numerically lower final BW compared to other treatments. Similar to the grower phase, gilts had lower BW, ADFI and FCR in the finisher phase. During the whole period, gilts showed lower final BW, ADFI, ADG and FCR compared to barrows.
Carcass characteristics
Carcass characteristics data are presented in Table 4. Back fat thickness was measured at day 70 of the trial and at slaughter, whereas all other parameters were measured at slaughter. No significant treatment effects were found on any of the carcass parameters. Muscle depth was numerically higher in phytase treatments compared to PC (P = 0.13). Gilts had lower (P < 0.05) back fat thickness, carcass weight, body fat and higher lean meat percentage compared to barrows.
Table 4. Effect of phytase on carcass characteristics in grower/finisher pigs slaughtered at 125 kg1

1 Each value represents the mean of 20 individually-housed pigs
2 Back fat thickness was measured at 70 days on trial (BW was not measured)
3 Back fat thickness and other parameters were measured at slaughter
Discussion
The first commercial microbial phytase for feed application was fungal-derived and marketed in early 90s. Since then, more efficient bacterial phytases have been developed and became commercially available to the feed industry (Lei et al., Reference Lei, Weaver, Mullaney, Ullah and Azain2013). The efficacy of commercial phytases differ significantly in in vivo conditions, i.e. at pH = 3 which represents the pH in the stomach of pigs. Studies have demonstrated that a Buttiauxella phytase has high activity at a lower and wider pH range, which is associated with a high phytate degradation rate (Menezes-Blackburn et al., Reference Menezes-Blackburn, Gabler and Greiner2015; Yu et al., Reference Yu, Cowieson, Gilbert, Plumstead and Dalsgaard2012; Reference Yu, Kvidtgaard, Isaksen and Dalsgaard2014). Therefore, the results from this study can be compared with literature studies where the same phytase was tested.
During the grower phase, increasing Buttiauxella phytase doses up to 1000 FTU/kg linearly improved ADG in pigs with a BW range of 30 to 85 kg and in a diet without inorganic phosphorus and with reduced levels of Ca and ME. The reduction in digestible P, Ca and ME in the NC diet reduced ADG by 2.6%, although this difference was not statistically significant. Interestingly, supplementation of 1000 FTU/kg Buttiauxella phytase to the P, Ca and ME deficient NC diet outperformed the PC (formulated to meet all requirements), whereby ADG improved by 5.3% and feed efficiency improvement by 2.9% during the grower phase of pigs with a BW range from 30–85 kg. Similarly, Remus et al. (Reference Remus, Dersjant-Li, Plumstead and Awati2015) showed that using the same phytase supplemented at 1000 FTU/kg to a NC diet (without inorganic P and reduced Ca and ME) outperformed a nutritionally adequate PC diet in grower pigs fed corn and soybean meal based diets. The improved performance with 1000 FTU/kg might be explained by the extra-phosphoric effect of high doses of Buttiauxella phytase. Lizardo et al. (Reference Lizardo, Dersjant-Li, Perez-Vendrell, Brufau, Owusu-Asiedu and Awati2015) observed that increasing the Buttiauxella phytase dose up to 2000 FTU/kg improved dry matter (DM) and nitrogen (N) digestibility in growing pigs, while Amerah et al. (Reference Amerah, Plumstead, Barnard and Kumar2014) reported that supplementation of Buttiauxella phytase at 1000 FTU/kg improved ileal AA digestibility on average by 10% in broilers. Similarly, with the same phytase, improvements in starch and protein digestibility were found in broilers (Liu et al., Reference Liu, Cadogan, Péronc, Truong and Selle2014, Reference Liu, Bold, Plumstead and Selle2015; Truong et al., Reference Truong, Bold, Liu and Selle2015). Bento et al. (Reference Bento, Pedersen, Plumstead, Salmon, Nyachoti and Bikker2012) reported that increasing Buttiauxella phytase dose up to 2000 FTU/kg not only improved apparent total tract digestibility of P and Ca, but also linearly improved DM digestibility in weaned piglets.
In the current study, data from the finisher phase from 85 to 125 kg BW showed no further improvement on performance with high doses of phytase at 1000 FTU/kg compared to the commonly used levels of 500 FTU/kg. This result may be explained by either the analysed 0.39 % total P in NC diet might be sufficient for finisher pigs – resulting, therefore, in no effect between PC and NC in finisher diet – or that pigs fed the NC diet had lower BW and tended to eat more to compensate growth during the finisher phase. Thus, it may be speculated that in the finisher phase, the 500 FTU/kg phytase had already broken down most of the phytate leaving little opportunity for further performance improvement. The analysed P values in finisher PC and NC diets were higher than formulated levels, which may be due to the increased by-product level in the finisher diets. Although the intrinsic phytase in basal diets was approximately 300 and 440 FTU/kg in NC grower and NC finisher diets respectively, this enzyme activity is less effective compared to applied exogenous phytase due to it's high pH optima (Von Sheuermann et al., Reference Von Sheuermann, Lantzoch and Marke1988). The Buttiauxella phytase used in this study has a high activity at around pH 3 (Menezes-Blackburn et al., Reference Menezes-Blackburn, Gabler and Greiner2015), and so would quickly degrade phytate in the stomach. Overall, the pigs had very high performance level (more than 1 kg body weight gain per day), which is close to the breeder-specified maximum genetic potential for the pigs used in this study.
Barrows had higher ADFI, ADG, final BW and FCR than gilts and the lower FCR may be explained by the reduced feed intake level. In agreement, Chen et al. (Reference Chen, Lewis, Miller and Yen1999) observed that barrows grew faster and utilised feed more efficiently than gilts during 0 to 35 d on trial (initial BW of 51 kg). In the finisher phase (35 to 75 d on trial), barrows consumed more feed but utilised it less efficiently than gilts. Similarly, Serrano et al. (Reference Serrano, Cámara, Morales, Berrocoso, López Bote and Mateos2013) reported that barrows had higher ADFI and ADG and better FCR than gilts (testing period from 19.4 to 110 kg), resulting in heavier slaughter weight.
Phytase supplementation had no significant impact on carcass characteristics, although muscle depth (longissimus dorsi) was 3 mm greater in pigs fed NC diet plus 1000 FTU/kg phytase treatment compared to PC. Lean meat percentage and back fat thickness were not influenced by phytase treatments. The results are in agreement with other literature studies, whereby phytase supplementation maintained carcass characteristics similar to the PC diet (Harper et al., Reference Harper, Kornegay and Schell1997; Shelton et al., Reference Shelton, Southern, LeMieux, Bidner and Page2004).
Gilts had higher lean meat and lower back fat thickness compared to barrows, confirming the data reported by Jaturasitha et al. (Reference Jaturasitha, Kamopas, Suppadit, Khiaosa-ard and Kreuzer2006), who observed that carcass fat percentage was significantly higher in barrows. Similarly, Serrano et al. (Reference Serrano, Cámara, Morales, Berrocoso, López Bote and Mateos2013) reported that carcass lean was 2.4% higher (P < 0.01) for gilts than for barrows.
The NC diet was formulated with less digestible P, Ca and ME, which led to a lower feed cost (using actual feed cost, with prices representative at time of trial) as shown in Table 1. Although statistically the differences between diets containing phytase at 1000 FTU/kg and the PC was non-significant, phytase at 1000 FTU/kg reduced FCR by seven points, resulting in considerable cost savings. Based on feed intake and diet price (including the cost price of phytase product), it was estimated that in the grower phase (30 to 85 kg BW), feed cost per kg weight gain was 0.773, 0.757, 0.744, 0.739 and 0.735€ for PC, NC, NC + 250, NC + 500 and NC + 1000 FTU/kg respectively. This resulted in a saving of 3.72, 4.46 and 4.92% on feed cost with phytase supplemented at 250, 500 and 1000 FTU/kg respectively, compared to PC. Thus increasing phytase dose from 500 to 1000 FTU/kg in grower pigs weighing 30 to 85 kg BW can result in additional economic benefits. Furthermore, using high dose phytase can further increase P digestibility and reduce P excretion, providing an environmental benefit (Adedokun et al. Reference Adedokun, Owusu-Asiedu, Ragland, Plumstead and Adeola2015; Bento et al., Reference Bento, Pedersen, Plumstead, Salmon, Nyachoti and Bikker2012).
Conclusions
The results from this study indicated that increasing Buttiauxella phytase dose up to 1000 FTU/kg in a complex diet based on multiple cereals (wheat, corn, barley and soybean meal) can improve weight gain and reduce FCR (above the commonly used dose of 500 FTU/kg) in the grower phase up to 85 kg body weight. However, no additional production performance benefit was observed with increasing phytase dose from 500 to 1000 FTU/kg during the finisher phase with pigs having BW range of 85–125 kg. The data from this study suggested that the optimal dose of phytase can be related to the age of pigs and dietary composition. With European-type complex diets containing different cereals with high amounts of intrinsic phytase such as those tested in this study, it can be still beneficial to use a high dose of 1000 FTU/kg Buttiauxella phytase in grower pigs up to 85 kg body weight. For finisher pigs above 85 kg body weight, the recommended dose would be 500 FTU/kg. These recommended levels can allow total replacement of inorganic phosphorus in the European-type complex diets.
Acknowledgments
The authors would like to acknowledge Danisco Animal Nutrition, DuPont Industrial Biosciences, Marlborough, UK for providing Axtra® PHY phytase. We recognize the care for the animals and assistance in conducting the experiments provided by the staff of the University of Applied Sciences, FB1- Life Sciences, Bingen am Rhein, Germany.
Declaration of Interest
Yueming Dersjant-Li, Alexandra Wealleans and Ajay Awati are employees of Danisco Animal Nutrition, DuPont Industrial Biosciences.








