Article contents
Synthesis, structure, and photoluminescence properties of Ce3+ and Tb3+ doped alkaline-earth silicate Sr2MgSi2O7 phosphors for WLEDs
Published online by Cambridge University Press: 12 January 2017
Abstract
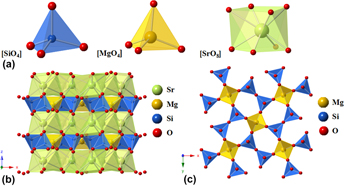
In this research, Ce3+ and Tb3+ doped alkaline-earth silicate Sr2MgSi2O7 phosphors have been synthesized by solid-state reaction. The results show that the Sr2−x MgSi2O7:xCe3+ phosphors exhibit a violet–blue emission with excitation at 348 nm, whereas the Sr2−y MgSi2O7:yTb3+ phosphor show a green emission with excitation at 243 nm. In addition, the structure of Sr2MgSi2O7 host has been analyzed by Crystalmaker program. Staggered arrangements of [SiO4] and [MgO4] units in the Sr2MgSi2O7 system underlie possible chemical tuning and phase segregation, providing a potential candidate of tunable luminescence. A red shift of wave length is clarified by crystal field theory and Van Uitert expression. The FESEM image of Sr1.99MgSi2O7:0.01Ce3+ phosphors reveal that it has a proper particle size for application in WLEDs. With different Tb3+ doping concentration, the CIE chromaticity coordinates Sr2MgSi2O7:Tb3+ phosphors still remain a steady position. These results indicate that Sr2−x MgSi2O7:xCe3+, Sr2−y MgSi2O7:yTb3+ phosphors are promising phosphors for WLEDs.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
References
REFERENCES
- 7
- Cited by





