Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder involving the progressive loss of motor neurons. In addition, cognitive impairments with a frontotemporal profile have been reported in 25%-50% of ALS patients, and about 5%-10% patients meet criteria for frontotemporal dementia (FTD).Reference Phukan, Elamin and Bede 1 However, identifying these changes in patients with ALS in clinic settings remains challenging, as the changes can be subtle and variable, and tests probing frontotemporal cognitive domains can be time-consuming and require examiner expertise—factors that are difficult to accommodate in a busy clinic.
To bridge this gap, screening tools are increasingly being used in clinics, especially in cognitive clinics.Reference Folstein, Folstein and McHugh 2 - Reference Nasreddine, Phillips and Bedirian 5 Screening tools have an advantage of assessing various domains of cognitive function as opposed to single tests, and they are time-efficient in a busy clinic setting. The Addenbrooke’s Cognitive Examination (ACE) was developed with the aim of distinguishing FTD from Alzheimer’s dementia (AD, 3). It included six domains with a maximum score of 100. The cut-off scores 88 and 83 have high sensitivity and specificity to cognitive impairment, respectively.Reference Mathuranath, Nestor, Berrios, Rakowicz and Hodges 3 In addition, a Verbal Fluency, Language, Orientation and Memory (VLOM) ratioFootnote a distinguished AD and FTD; in participants with a VLOM ratio ≥ 3.2, there is a 76% probability of AD, whereas those with VLOM ratio ≤ 2.2 have a 71% probability of FTD.
Considering the sensitivity of the ACE to frontotemporal changes,Reference Mathuranath, Nestor, Berrios, Rakowicz and Hodges 3 our retrospective cross-sectional study evaluated the utility of the ACE for ALS patients. Our aims were to (i) compare performance of patient and control groups on the ACE; (ii) evaluate the performance on the ACE against formal neuropsychometric tests; and (iii) identify associations between the ACE and clinical variables such as disability, respiratory function and finger tapping. We hypothesised that patients would perform poorly on the ACE as compared with controls. We hypothesised that the ACE would have construct validity as indicated by associations between scores on the ACE and the neuropsychometric tests.Reference Galton, Erzinclioglu, Sahakian, Antoun and Hodges 6 Finally, considering the motor components of some tasks on the ACE, we hypothesised that factors such as motor disability would be associated with poor ACE scores.
Methods
Sample
The ACE data in the present study were collated from five studies initiated by the ALS Research Program between 2006 and 2008 at the University of Alberta. To ensure minimal bias during data collection, neuropsychometric training was provided by a single psychometrician for test administration and scoring. This training was completed by research assistants and students involved in the studies. The neuropsychometric protocol was kept consistent for participants within each study. All studies were approved by the Health Research Ethics Board, and a formal consent was obtained from all participants for the corresponding studies. The ACE was administered on 59 ALS patients meeting criteria for possible, probable or definite ALS;Reference Brooks, Miller, Swash and Munsat 7 five were unable to complete the ACE owing to motor deficits and were excluded from further analysis. Our final sample included 54 patients (Table 1). Patients had moderate to low disease burden as reflected by their ALS Functional Rating Scale-Revised (ALSFRS-RReference Cedarbaum, Stambler and Malta 8 ) and forced vital capacity (FVC). In total, 47 healthy volunteers without a history of neurological or psychiatric conditions were included (Table 1). Differences in education were accounted for in the statistical model used for analysis.
Table 1 Participant demographics

ALSFRS-R=ALS Functional Rating Scale-Revised; FVC=forced vital capacity.
* Available in 52 patients.
Instrument
The ACE evaluates six aspects of cognition: orientation, attention, memory, verbal fluency, language and visuospatial ability, and incorporates items from the Mini-Mental State Examination (MMSE).Reference Folstein, Folstein and McHugh 2 , Reference Mathuranath, Nestor, Berrios, Rakowicz and Hodges 3 The total administration time is approximately 15-20 minutes.
Neuropsychometric Tests
A subset of 35 ALS patients and 41 healthy controls were administered additional neuropsychometric tests assessing additional cognitive domains such as executive functions, language, learning and memory, and visuospatial abilities. In total, 23 patients and 17 controls completed digit span (forward and backward) from Wechsler Adult Intelligence Scale-Revised (WAIS-R),Reference Wechsler 9 digit ordering test,Reference Werheid, Hoppe, Thone, Muller, Mungersdorf and von Cramon 10 Boston Naming Test (BNT)Reference Mack, Freed, Williams and Henderson 11 and Benton Judgement of Line Orientation.Reference Benton, Varney and Hamsher 12 In total, 21 participants also completed the Stroop Test,Reference Stroop 13 , Reference Golden 14 and a smaller sub-group completed Wechsler’s Test of Adult ReadingReference Wechsler 15 ) and the California Verbal Learning Test-II (CVLT-IIReference Delis, Kramer and Kaplan 16 ). In total, 12 patients and 24 controls were administered only verbal fluency (letter F and AReference Borkowski, Benton and Spreen 17 , Reference Spreen and Benton 18 ). Mood was assessed using the Beck’s Depression Inventory-II (BDI-II) in 19 patients and 17 controls (see Supplementary Table 4).
Statistical Analysis
IBM SPSS software package (version 21) was used for analysis. 19 Normality was tested for the ACE and neuropsychometric tests using either Shapiro-Wilk test or Kolmogorov-Smirnov tests (Supplementary Tables 1 and 2). Analysis of covariance with education as a covariate was used to compare group differences in performance on the ACE. The percentage of patients and controls below (a) published cut-off scores and (b) derived cut-off scores (5th percentile of the control group) were calculated and the distribution of participants was compared using Pearson’s χ2 test. Spearman’s correlations were calculated between the ACE and neuropsychometric tests for each group of participants. Associations between the ACE and clinical variables were also computed for patients. Statistical significance was accepted at p<0.05 and corrections for multiple comparisons (false discovery rate, FDR) were performed where applicable.
Results
Group Comparisons
The patient and control groups were matched for age (p>0.05). However, they differed significantly for education and gender distribution (Table 1). There were no significant differences in performance on the ACE based on gender; thus, only education was used as a covariate in further group comparisons. There were no significant differences in performance on the total ACE score individual domains between patients and controls, except for a strong trend in the visuospatial ability of the ACE (Supplementary Table 3). Neuropsychometric tests indicated a significant difference for BDI-II (p<0.01, FDR-corrected). At more lenient thresholds, digit ordering test (p=0.05, uncorrected) and Stroop non-interference condition (words, p<0.01 uncorrected) were significantly lower as compared with controls. Judgement of line orientation was lower in controls as compared with patients (p=0.04, uncorrected).
Participants Below Cut-Off Scores
A higher percentage of patients were below cut-off scores as compared with controls, although this was not statistically significant (Figure 1). The published cut-off scores were proposed by Mathuranath et al,Reference Mathuranath, Nestor, Berrios, Rakowicz and Hodges 3 whereas our derived cut-off scores are the 5th percentiles of our control group.
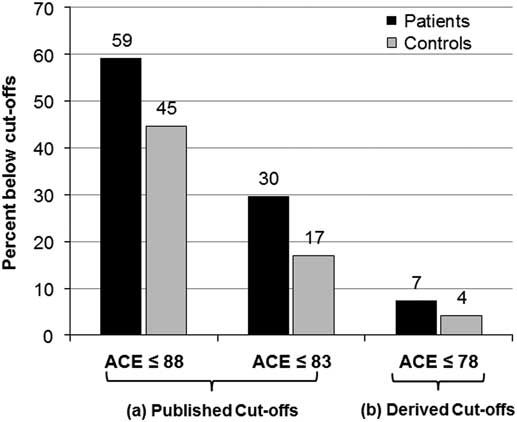
Figure 1 Percentage of patients and controls below cut-off scores on the Addenbrooke’s Cognitive Examination (ACE). There were no significant differences in the distribution of patients and controls below (A) the published cut-off scores of ACE ≤ 88 for high sensitivity (χ 2=2.7, p=0.09) or ACE ≤83 for high specificity (χ 2=2.5, p=0.11), and (B) the derived cut-off score ACE ≤ 78 (χ 2=0.5, p=0.47).
ACE and the Neuropsychometric Battery
Spearman’s correlations of the ACE and performance on neuropsychometric tests for each participant group indicated moderate-to-strong positive associations of ACE Total Score memory and verbal fluency domains with verbal fluency (letter F and A; Supplementary Table 5). Higher ACE Total indicated a significant association with lower language (BNT) performance in controls (p<0.05, uncorrected), but not patients. A significant association with higher ACE Total, memory and CVLT short-delay recall was noted in patients, but not controls (p<0.05, uncorrected). Language scores on the ACE did not show any associations with Boston Naming Test for patients or controls. Visuospatial abilities did not reveal any significant associations either. Higher VLOM ratios were associated with lower performance on CVLT short-delay recall for controls (p<0.05, uncorrected), but not patients. CVLT long-delay recall showed trends with ACE Total, MMSE and VLOM ratios in controls (p<0.09) and only with VLOM ratio in patients (p<0.09). Non-meaningful associations were noted between ACE orientation, judgement of line orientation and ACE memory and Stroop interference performance in controls.
ACE and Clinical Variables
Spearman’s correlations with clinical variables indicated that lower ALSFRS-R was moderately associated with lower ACE Total Score (Spearman’s ρ=0.6, p<0.01; Figure 2A) and lower memory score (Spearman’s ρ=0.5, p<0.05). A significant association was also noted between finger-tapping and ACE Total performance (Spearman’s ρ=0.5, p<0.05; Figure 2B). There was a trend towards associations between lower ALSFRS-R and poor ACE Verbal Fluency score (Spearman’s ρ=0.4, p=0.07; Supplementary Figure). There were no significant correlations between performance on the ACE and FVC.
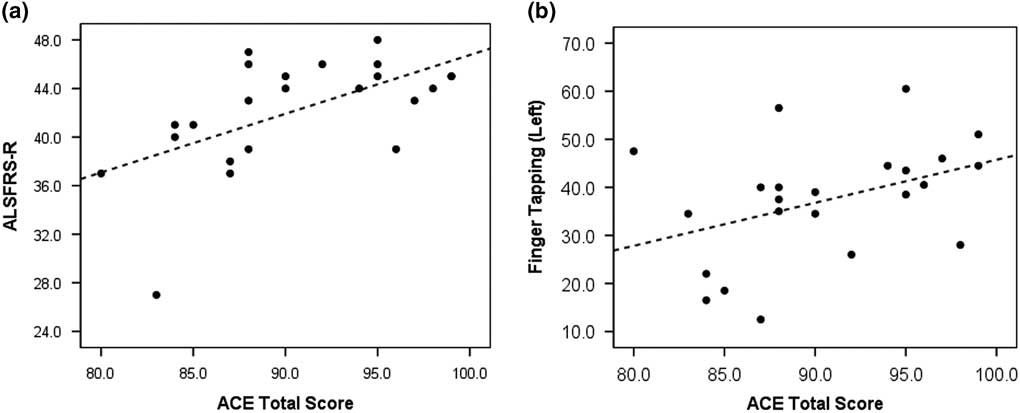
Figure 2 Scatterplot graphs indicating linear associations between (A) amyotrophic lateral sclerosis Functional Rating Scale-Revised (ALSFRS-R) and Addenbrooke’s Cognitive Examination (ACE) Total Score (Spearman’s ρ=0.6, p<0.01), and (B) Finger-tapping and ACE Total score (Spearman’s ρ=0.5, p<0.05).
Discussion
Our study evaluated the utility of the ACE as a screening tool for ALS. We investigated this by (i) comparing performances of ALS patients and healthy controls, (ii) validating the ACE against a standard neuropsychometric battery and (iii) associating performance on the ACE with clinical variables such as the ALSFRS-R.
Group Comparisons and Per cent Below Cut-Off Scores
We report no significant differences in performance between patients and controls on the ACE. A strong trend was noted in visuospatial ability, with controls performing lower than patients; however, this was driven by 1 outlier. The trend was eliminated when the control was excluded from analysis. Although a higher proportion of patients scored below published and derived cut-off scores on the ACE, there were no significant differences in the distribution of participants below (a) published and (b) derived cut-off scores. To the best of our knowledge, this is the only report of the use of the original ACE in ALS. We acknowledge that the ACE is an older test while recent revised versions have been used in the literature. Studies have emerged reporting the application of the revised version of the ACE (ACE-R)Reference Mioshi, Dawson, Mitchell, Arnold and Hodges 20 in ALS to either identify cognitive impairment in the ALS-FTD spectrumReference Lillo, Savage, Mioshi, Kiernan and Hodges 21 - Reference De Silva, Hsieh and Caga 25 or as a screening tool for recruitment.Reference Staios, Fisher, Lindell, Ong, Howe and Reardon 26 The ACE-R incorporates content changes to the domains of memory, language and visuospatial abilities of the ACE to ensure a more distributed scoring across the domainsReference Mioshi, Dawson, Mitchell, Arnold and Hodges 20 and improved diagnostic accuracy.Reference Larner and Mitchell 27 Our recruitment began before the publication of the ACE-R, when there were limited cognitive screening tools and no specific cognitive tests for ALS. The ACE provided the option for frontotemporal assessment, much needed for evaluating the frontotemporal lobar degeneration in ALS. We continued using the original ACE to maintain consistency between studies.
Across the ALS-FTD spectrum, there are inconsistent reports of impairment using the ACE-R. One study reported comparable performance on the ACE-R between ALS and behavioural variant FTD patients; both groups performed poorly as compared with controls.Reference Lillo, Savage, Mioshi, Kiernan and Hodges 21 The study included 5 of 20 ALS patients meeting criteria for FTD, which explains the comparable performance between the patient groups given the small sample sizes. In contrast, another study reported poor ACE-R scores in ALS-FTD patients, and comparable scores between non-demented ALS patients and healthy controls.Reference Savage, Lillo, Kumfor, Kiernan, Piguet and Hodges 22 Using the ACE-R semantic deficits have also been reported in ALS patients with a graded performance as compared with other groups; order of language score from highest to lowest is controls, followed by ALS, ALS-FTD and semantic dementia patients.Reference Leslie, Hsieh and Caga 23 Higher age, female gender and lower education have been reported as potential determinants of cognitive impairments in ALS based on ACE-R scores.Reference Wei, Chen and Zheng 24
A recent study by Hsieh et alReference Hsieh, Caga and Leslie 28 utilised the mini Addenbrooke’s Cognitive Examination version-III (M-ACE). The M-ACE included items assessing orientation in time, executive functions (animal fluency), visuospatial abilities (clock drawing) and learning and recall of an address.Reference Hsieh, McGrory and Leslie 29 It was used in combination with Motor Neuron Disease Behavioural Scale (MiND-B)Reference Mioshi, Hsieh and Caga 30 to determine cognitive and behavioural changes in the ALS-FTD spectrum. The authors reported that 90% of ALS-FTD patients and only 20% of non-demented ALS patients scored below cut-offs for the M-ACE, whereas the MiND-B questionnaire distinguished ALS patients with behavioural or cognitive impairments from non-demented ALS. Although recent versions of the ACE may have provided an advantage in identifying cognitive impairments, the studies include a wide range of cognitive profiles in ALS patients, with higher prevalence of ALS-FTD patients. In addition, language and memory domains contribute to 50% of the memory score in ACE-R and ACE-III. These two domains include motor-based tasks that would require careful implementation of corrections for motor impairments. Although this is not a direct comparison, it remains a cautionary note given the construct of the ACE.
ACE Scores and the Neuropsychometric Battery
Higher scores on the ACE Total, memory and verbal fluency domains were associated with corresponding tests on the neuropsychometric battery, suggesting construct validity of these ACE domains. It was surprising to see no association between the language scores on the ACE and the Boston Naming Test or between visuospatial abilities and Judgement of Line Orientation. One possibility is that these tests were performed on a small subset of participants and hence may lack sufficient power in our analysis. Additional details outlining the associations are included in Supplementary Table 5.
ACE Scores and Clinical Variables
Poor ACE scores for verbal fluency were associated with lower ALSFRS-R (greater disability), indicating that patients with greater motor dysfunctions perform poorly on the ACE. One possibility is that patients with motor dysfunction indeed had cognitive impairments.Reference Bak and Chandran 31 Alternatively, we could consider poor performance on the ACE to be associated with reduced motor ability to perform the task, and not associated with cognitive impairment. This raises concern regarding the ability of the ACE to identify cognitive impairment independent of motor dysfunctions.Reference Abrahams, Leigh, Harvey, Vythelingum, Grise and Goldstein 32 Anecdotal reports by Abrahams et alReference Abrahams 33 indicated that only 59% of ALS patients completed all the domains of the ACE-R. The domains that were completed differed among patients owing to their varying range of disabilities and thereby restricted comparisons.
More recent versions of the ACE have been published since our last data collection. ACE-III and M-ACE are reported to be more sensitive in screening for cognitive impairments in clinical populations.Reference Hsieh, Schubert, Hoon, Mioshi and Hodges 34 , Reference Larner 35 These versions still require modifications to account for motor disability and do not address social cognition and behavioural changes in ALS. Studies also report utility of ALS-specific tools such as the ALS Cognitive Behavioural ScreenReference Woolley, York and Moore 36 and Edinburgh Cognitive and Behavioural ALS Screen (ECAS)Reference Abrahams, Newton, Niven, Foley and Bak 37 and would be of interest for consideration in ALS clinics. A recent study compared performance of ALS patients and neuromuscular disease controls on cognitive screening tools (ACE-III, Frontal Assessment Battery [FAB] and ECAS executive domain). The authors report significantly lower performance on total scores of the ACE-III and the FAB for ALS patients.Reference Xu, Alruwaili, Henderson and McCombe 38 Impairment was identified in 30% of ALS patients (n=81) on the ACE-III, 14% on the FAB and 22% on the ECAS executive domain (n=41). Motor impairments in that study were corrected for by converting scores into per cent correct responses. However, the authors do not reveal whether patients impaired on ACE-III were also impaired on the FAB or ECAS executive domain.
In conclusion, we report non-significant differences in performance on the ACE in patients compared with controls. Although some of the ACE domains were positively associated with neuropsychometric battery, indicating construct validity, other domains such as language and visuospatial abilities showed poor or no associations with the neuropsychometric battery. The association between motor disability and poor ACE scores raises concern for its clinical utility in ALS. Although recent versions of the ACE such as the M-ACE have been explored in combination with behavioural screens, its utility in ALS clinics where patients show a varying range of motor dysfunctions needs further validation. We did not assess behaviour in our sample, whereas mood was assessed for a smaller subset; evaluation of these domains should be considered for validating other ALS-specific tools.
Acknowledgements
The authors sincerely thank patients and controls for taking time and participating in our studies.
Disclosures
All authors have no conflicts of interest to disclose.
Statement of Authorship
SC was involved with collating ACE data, designing experiments for data analysis, interpretation and writing the manuscript. DM was involved in data collection and collating ACE data. WJ provided expert advice on data analysis, interpretation and editing the manuscript. RC and NF were involved in designing the core neuropsychometric battery in our studies. SK, the principal investigator, was involved in designing the experiments, provided advice on data analysis and editing the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.68







