Introduction
Human dirofilariosis is the zoonotic variety of the animal dirofilariosis caused by the species of the genus Dirofilaria, which are parasitic filarial nematodes distributed throughout the tropical and temperate regions of the world (Simón et al., Reference Simón, Siles-Lucas, Morchón, González-Miguel, Mellado, Carretón and Montoya-Alonso2012). Domestic and wild canids are the reservoirs of this infection, in which circulating microfilariae are ingested by culicid mosquitoes and transmitted to the final host after two consecutive moults to the infective third-stage larvae (L3). During this cycle, humans can be accidental hosts of Dirofilaria spp. when they are bitten by infected mosquitoes (McCall et al., Reference McCall, Genchi, Kramer, Guerrero and Venco2008).
Clinical presentation of human dirofilariosis includes pulmonary and subcutaneous/ocular infections, which have been classically ascribed to Dirofilaria immitis and Dirofilaria repens species, respectively. In most cases, these infections cause the development of benign pulmonary and/or subcutaneous nodules, which are mainly attributed to the inflammatory reaction against dying worms that cannot reach maturity in this host. However, clinical cases caused by mature worms, sometimes accompanied by circulating microfilariae, as well as infections involving serious medical complications, are increasingly being reported (Simón et al., Reference Simón, Siles-Lucas, Morchón, González-Miguel, Mellado, Carretón and Montoya-Alonso2012; Ilyasov et al., Reference Ilyasov, Kartashev, Bastrikov, Madjugina, González-Miguel, Morchón and Simón2015).
Although distribution of the disease appears to be cosmopolitan, human dirofilariosis cases are not homogenously reported throughout the world. According the latest estimates, one third of the human cases of dirofilariosis have been reported in the Russian Federation (more than 1400 cases) (Ermakova et al., Reference Ermakova, Nagorny, Krivorotova, Pshenichnaya and Matina2014; Kartashev et al., Reference Kartashev, Tverdokhlebova, Korzan, Vedenkov, Simón, González-Miguel, Morchón, Siles-Lucas and Simón2015; ESDA, 2017; Moskvina & Ermolenko, Reference Moskvina and Ermolenko2018; Kondrashin et al., Reference Kondrashin, Morozova, Stepanova, Turbabina, Maksimova and Morozov2020). However, it seems that only ‘the tip of the iceberg’ is portrayed since clinical cases only represent a part of human infections, and most cases remain asymptomatic, especially those cases that result in pulmonary nodules. Notwithstanding this, humans usually demonstrate a strong antibody response against Dirofilaria spp. For that reason, seroepidemiological studies performed in human populations living in endemic areas are useful to study their exposure to infection, as well as to evaluate the risk of transmission. Interestingly, available human dirofilariosis prevalence data show that the incidence of infection in humans is close to that observed in canine populations of the same areas (Simón et al., Reference Simón, Muro-Alvarez, Cordero-Sánchez and Martín-Martín1991, Reference Simón, Siles-Lucas, Morchón, González-Miguel, Mellado, Carretón and Montoya-Alonso2012).
The objective of this work is to compare the seroprevalence of Dirofilaria antibodies in totally random donors from five different regions distributed throughout the Russian Federation in order to obtain information on the real risk of dirofilariosis infection in this country.
Materials and methods
Description of the study area
Five regions distributed throughout the Russian Federation were included in the study. From west to east, these were Rostov, Moscow, Ekaterinburg, Yakutia and Khabarovsk (fig. 1). The Rostov region is located in the south-west of the Russian Federation, and has an area of 100,800 km2 and a population of 4,277,976. The Moscow region (territory around the federal city of Moscow) has an area of 44,300 km2 and a population of 7,095,120. The Ekaterinburg region is located at the border of the European and Asian parts of Russia, has an extension of 194,800 km2 and a population of 4,297,747. The Yakutia region is located in the Far Eastern Federal District, it is the largest subnational governing body by area in the world at 3,083,523 km2 and has a population of 958,528. Finally, the Khabarovsk region, which is located in the Far East region of the country, has an extension of 788,600 km2 and a population of 1,343,869 (Russian Census, 2010).
Fig. 1. Geographical localization of the administrative regions of the Russian Federation included in the study. Abbreviations: R, Rostov region; M, Moscow region; E, Ekaterinburg region; Y, Yakutia region; K, Khabarovsk region.
All regions ranging from humid continental climate with clearly expressed seasonality in Rostov, Moscow and Ekaterinburg (short but warm summers and long, cold winters) to hyper-continental climate with extremely strong annual swings (hot, wet and humid summers, which rapidly transform into severely cold and long winters) in Yakutia and Khabarovsk. Climatic data of yearly average temperature (°C) and rainfall (mm) for each region were calculated within the period 1952–2012 following databases published by Bulygina et al. (Reference Bulygina, Veselov, Razuvaev and Aleksandrova2014). These data were: Rostov (9.7°C, 598.5 mm), Moscow (5.3°C, 676.4 mm), Ekaterinburg (2.1°C, 512.1 mm), Yakutia (−10.3°C, 216.2 mm) and Khabarovsk (2.2°C, 688.3 mm) (table 1). In addition, and according to the climatic model developed for the Russian Federation by Kartashev et al. (Reference Kartashev, Afonin, González-Miguel, Sepúlveda, Simón, Morchón and Simón2014) based on temperature data, regions included in this study were predicted to have the following number of yearly generations of Dirofilaria: Rostov (between two and six in all of the territory); Khabarovsk (between one and six in the southern half of the territory); Moscow (between one and two in all of the territory); and Ekaterinburg and Yakutia (between one and two in a few spots of the territory).
Table 1. Dirofilaria immitis seroprevalence data of human serum samples, as well as yearly average temperature (°C) and rainfall (mm) from the Russian regions of Rostov, Moscow, Ekaterinburg, Yakutia and Khabarovsk.
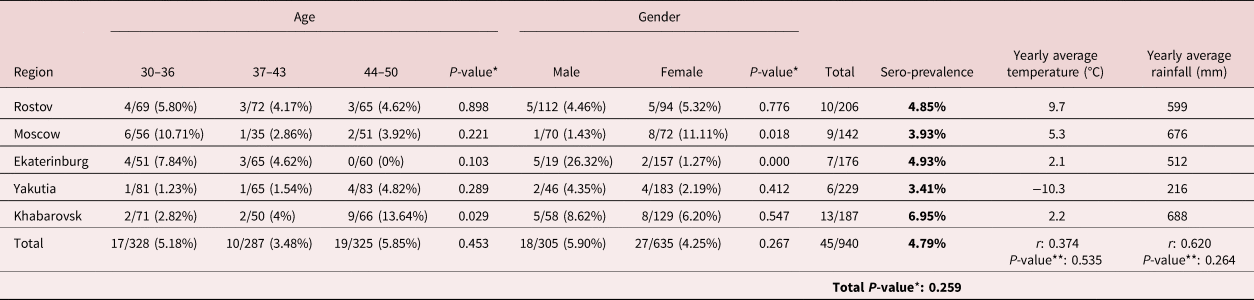
Results are expressed as number of positive samples/number of samples analysed (seroprevalence percentage). *P-value shows statistical association between categorical variables (Dirofilaria exposure, age, gender and region) performed by Pearson's chi-squared test. **P-value shows statistical correlation between D. immitis seroprevalence and yearly average temperature (°C) or rainfall (mm) data performed by Pearson's correlation coefficient (r). The level of significance was set at P ≤ 0.05. Climatic data were obtained by accession to dataset of the main meteorological parameters at the Russian Weather Stations (Bulygina et al., Reference Bulygina, Veselov, Razuvaev and Aleksandrova2014).
All regions except for Rostov have a developed wild berries and nuts picking industry (in decreasing order, Khabarovsk–Yakutia–Ekaterinburg–Moscow region). Hunting is most common in Yakutia (over 11% of population are registered hunters), closely followed by Khabarovsk region, while in other regions the proportion of hunters among population is much lower. Khabarovsk also has the most developed lumber industry among the study regions.
Sampling of the human serum samples
A totally random sample of sera were obtained following the principles explained by Tyrer & Heyman (Reference Tyrer and Heyman2016) for sampling in epidemiological research. Thus, donors between 30 and 50 years old living in the five selected regions were randomly asked to participate in this study. All patients were properly informed, and they gave their consent to participate in the study. A total of 940 serum samples (305 males and 635 females) were collected from Rostov (206), Moscow (142), Ekaterinburg (176), Yakutia (229) and Khabarovsk (187) regions (table 1). Serum samples were collected as a part of hepatitis B seroprevalence study (Klushkina et al., Reference Klushkina, Kyuregyan, Kozhanova, Popova, Dubrovina, Isaeva, Gordeychuk and Mikhailov2016). Serum collection was approved by the Ethics Committee of the Chumakov Institute of Poliomyelitis and Viral Encephalitides, Moscow, Russia (approval #6 from 2010-04-01).
Anti-D. immitis antibodies detection
Detection of the specific immunoglobulin G (IgG) antibody response against D. immitis was analysed in human serum samples by a non-commercial enzyme-linked immunosorbent assay (ELISA) test, as previously described by Simón et al. (Reference Simón, Muro-Alvarez, Cordero-Sánchez and Martín-Martín1991), with some modifications. In brief, a somatic extract of D. immitis adult worms (DiSA) was employed to coat Costar® 96-well microplates (Corning, New York) with 0.8 μg of antigenic extract per well in carbonate buffer (pH = 9.6) (final volume: 200 μl/well) overnight at 4°C. The wells were blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and incubated successively with human serum samples at 1:100 dilution and then with a peroxidase-conjugated rabbit anti-human IgG (Sigma-Aldrich, St. Louis, Missouri) at 1:4000 dilution. All incubations were performed at 37°C and, between each step, plates were washed three times with PBS washing buffer (PBS containing 0.05% Tween20). Ortho-phenylene diamine was used as a chromogen and reaction was stopped with sulphuric acid 3N. Optical densities (ODs) were measured at 492 nm in Easy Reader (Bio-Rad Laboratories, Hercules, California, USA). Samples were analysed in duplicate in at least two independent experiments for each sample. The cut-off (OD = 1.2) was re-established from previous studies using a similar methodology by calculating the mean value +3 standard deviation of 40 serum samples from clinically healthy blood donors (negative controls) without travel history living in a dirofilariosis-free area (some parts of Yakutia region) according to the Dirofilaria transmission model developed by Kartashev et al. (Reference Kartashev, Afonin, González-Miguel, Sepúlveda, Simón, Morchón and Simón2014).
Statistical analyses
Statistical association between categorical variables (Dirofilaria exposure, age, gender and region) was performed by Pearson's chi-squared test. Statistical correlation between Dirofilaria seroprevalence and climatic data (yearly average temperature and rainfall) was performed by Pearson's correlation analysis. The level of significance was set at P ≤ 0.05. Data were analysed using SPSS Base 20.0 software for Windows (SPSS Inc./IBM, Chicago, Illinois, USA).
Results and discussion
Although in most cases human dirofilariosis does not represent a significant health problem due to the activity of host immune response preventing the complete development of the parasites, human cases involving serious medical consequences, especially those reaching ocular locations, are being increasingly reported (Simón et al., Reference Simón, Siles-Lucas, Morchón, González-Miguel, Mellado, Carretón and Montoya-Alonso2012; Ilyasov et al., Reference Ilyasov, Kartashev, Bastrikov, Madjugina, González-Miguel, Morchón and Simón2015). Nevertheless, human exposure to the parasite is enough to stimulate the production of specific antibodies by the immune system of the host, which means that seroepidemiological studies can help us to evaluate the risk of zoonotic transmission of Dirofilaria spp. The Russian Federation is currently second from the top (only behind Ukraine) in terms of human dirofilariosis incidence (ESDA, 2017). However, epidemiological studies determining the real exposure of population to the parasite are scarce in this country. For this reason, the aim of this study was to evaluate the potential risk of Dirofilaria spp. transmission to the human population in five different regions of the Russian Federation by using a seroepidemiological approach.
First of all, we decided to use a totally random sample of sera according to the recommendations for sampling in epidemiological research (Tyrer & Heyman, Reference Tyrer and Heyman2016). Secondly, we employed a non-commercial ELISA for the detection of Dirofilaria-specific antibodies based on a serological detection of a DiSA extract, whose usefulness had previously been demonstrated in similar studies performed in other European countries to determine seroprevalence of Dirofilaria spp. in the human population (Morchón et al., Reference Morchón, Moya, González-Miguel, Montoya and Simón2010; Montoya-Alonso et al., Reference Montoya-Alonso, Carretón, Corbera, Juste, Mellado, Morchón and Simón2011; Ciuca et al., Reference Ciuca, Simón, Rinaldi, Kramer, Genchi, Cringoli, Acatrinei, Miron and Morchon2018). Despite the fact that crude antigens can cross-react among different Dirofilaria species and with other parasitic helminths of humans (Simón et al., Reference Simón, Muro-Alvarez, Cordero-Sánchez and Martín-Martín1991), a recent validation performed in a diagnostic study including clinically diagnosed patients with dirofilariosis, toxocariasis, giardiasis, as well as healthy controls, showed diagnostic accuracy of 83% for a similar ELISA assay (Ermakova et al., Reference Ermakova, Nagorny, Krivorotova, Pshenichnaya and Matina2014). Furthermore, according to the guidelines for clinical management of human Dirofilaria infection issued by the European Society of dirofilariosis and Angiostrongylosis (ESDA), despite being considered as a complementary technique in order to make an accurate diagnostic, the detection of Dirofilaria antibodies, no matter which species of the genus is involved, is useful for determining the risk of infection and transmission in different geographical areas and are important for epidemiological studies (ESDA, 2017).
Dirofilaria immitis seroprevalence data are shown in table 1. Forty-five out of the 940 human serum samples analysed were positive (4.79%). Seroprevalence among regions included in this study were 4.85% (Rostov), 3.93% (Moscow), 4.93% (Ekaterinburg), 3.41% (Yakutia) and 6.95% (Khabarovsk), revealing no statistical differences among regions (P-value: 0.259). Some of these regions are geographically very large. Therefore, the fact of not considering specific locations of each sample within them may be considered as a limitation of this and other previous studies (Morchón et al., Reference Morchón, Moya, González-Miguel, Montoya and Simón2010; Montoya-Alonso et al., Reference Montoya-Alonso, Carretón, Corbera, Juste, Mellado, Morchón and Simón2011; Ciuca et al., Reference Ciuca, Simón, Rinaldi, Kramer, Genchi, Cringoli, Acatrinei, Miron and Morchon2018), which should be improved in future seroprevalence assays. Based on a classical approach of the study of the factors influencing Dirofilaria transmission patterns, mainly climatic factors (temperature and humidity), it have been expected to find a higher seroprevalence data in the warmer regions of south-western Russia, such as Rostov region. However, no significant correlation between obtained Dirofilaria seroprevalence and yearly average temperature (r: 0.374; P-value: 0.535) and rainfall (r: 0.620; P-value: 0.264) among studied regions were found (table 1). On the other hand, modern approaches take into account many other factors influencing the spread of Dirofilaria. The anthropogenic influence on the natural environment can have epidemiological consequences, such as the presence of irrigated areas or the habitat modification, as well as the human behaviour related to travels and pet management, among others (Simón et al., Reference Simón, González-Miguel, Diosdado, Gómez, Morchón and Kartashev2017). Consequently, it could be difficult to draw epidemiological conclusions on the human population without having data on all the factors influencing transmission.
Another point worth noting is that despite the climatic differences among studied regions, according to published climatic models analysing the influence of regional warming on the geographical spreading and potential risk of infection of human dirofilariosis in Russia, at least a yearly generation of Dirofilaria is predicted in each region included in our study (Kartashev et al., Reference Kartashev, Afonin, González-Miguel, Sepúlveda, Simón, Morchón and Simón2014). This assumes that all these regions can potentially reach the 130 Dirofilaria growing degree-days above 14°C proposed to be required for the extrinsic development into the infective stage in mosquitoes (Rinaldi et al., Reference Rinaldi, Musella, Biggeri and Cringoli2006; Genchi & Kramer, Reference Genchi and Kramer2019). In addition, global warming is enabling the spread of vector-borne diseases, such as dirofilariosis, to locations as northern as Finland (Pietikäinen et al., Reference Pietikäinen, Nordling and Jokiranta2017). Regarding Dirofilaria vectors, a recent study involving molecular analyses in more than 5000 mosquitoes collected in central European Russia and on the Black Sea coast showed similar Dirofilaria estimated infection rates (around 3%). Authors concluded that factors other than temperature – for example, the presence of infected dogs – could have a greater effect on the maintenance of dirofilariosis foci (Shaikevich et al., Reference Shaikevich, Bogacheva and Ganushkina2019). Additionally, the presence of potentially competent reservoirs for Dirofilaria might be insured in all the studied regions since epidemiological studies performed in dogs have denounced prevalence ranging from 3.6% (Moscow region) to 36–55% in the southern and central areas of the country and 43.6% in far eastern regions (Genchi & Kramer, Reference Genchi and Kramer2019). Although information on the extension of dirofilariosis in wild reservoirs is scarce, it seems that wild canines could be acting as potential reservoirs of the parasite influencing its transmission in a country with a great tradition of hunting and picking forest berries, mushrooms and nuts. Nevertheless, more studies must be carried out in order to understand the real risk of these wild infections for humans (Kravchenko et al., Reference Kravchenko, Itin, Kartashev, Ermakov, Kartashov, Diosdado, González-Miguel and Simón2016).
Considering age and gender distribution, the highest seroprevalence was observed in the 44–50 years age group (19/325 (5.85%)), followed by the 30–36 (17/328 (5.18%)) and the 37–43 years age group (10/287 (3.48%)). In addition, 18 out of 305 males (5.90%) and 27 out of 635 serum samples from females (4.25%) had a positive reaction to D. immitis ELISA. Statistical analyses revealed neither age nor gender influence on Dirofilaria exposure (P-values: 0.453 and 0.267, respectively) for the combined data for five regions. On the contrary, statistically significant associations were found within regions. There were significant associations between Dirofilaria seropositivity and the 44–50 years age group in Khabarovsk (13.64%; P-value: 0.029), the female group in Moscow (11.11%; P-value: 0.018) and the male group of patients in Ekaterinburg (26.32%; P-value: 0.000) (table 1). According to the ESDA guidelines for clinical management of human dirofilariosis, the infection is most frequently described in patients around 40 years old, but the age of the patients can vary, mostly from 21–40 to 41–60 years. Regarding gender, larger studies have demonstrated that women are much more often affected than men. The proportion of female to male patients, definitely established from the larger study, ranges from 67.4% to 74.4%, with a clear predominance of females (ESDA, 2017). This corresponds with the observed trend in seroprevalence in Moscow region, but it is the opposite of what was shown by patients from Ekaterinburg region (table 1). Nevertheless, and despite this apparent contradiction, human behaviour can influence the number of times that someone can be bitten by a mosquito (Simón et al., Reference Simón, González-Miguel, Diosdado, Gómez, Morchón and Kartashev2017). Consequently, it would be necessary to find a behavioural explanation for the high percentage of positive men in Ekaterinburg – for example, the hunting and fishing activities practised by men in this region, which could facilitate contact with water bodies and humid areas with vegetation cover, where vector populations are likely to be highest.
In conclusion, we have performed a human seroepidemiological study in five different regions of the Russian Federation, with the aim to evaluate the risk of zoonotic transmission of Dirofilaria throughout these territories. Results showed that despite the climatic differences among studied regions, similar D. immitis seroprevalence data have been found. This could indicate that although dirofilariosis is a vector-borne disease that surely depends on climatic factors that ensure the presence of competent vectors and development of the parasite in them, other poorly quantifiable factors related to human activity and behaviour could define exposure to the parasite and shape the epidemiological profile of the overt disease.
Acknowledgements
We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Financial support
This work was supported by the Russian Academic Excellence Project 5-100.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






