Conjugated linoleic acid (CLA) refers to a group of dienoic derivatives of linoleic acid with conjugated double bonds arranged in different combinations of cis and trans configuration( Reference Pariza, Park and Cook 1 ). The bioactive properties of c9t11-CLA and t10c12-CLA isomers have been extensively studied. CLA is incorporated into tissues( Reference Kelley, Bartolini and Newman 2 ), increases energy expenditure, modulates TAG metabolism in liver, adipose tissue and skeletal muscle, modifies inflammatory markers and regulates oxidative status( Reference Bhattacharya, Banu and Rahman 3 , Reference Kim, Kim and Ahn 4 ). Studies in different experimental animal models have shown controversial results on hepatic TAG content. CLA might attenuate or prevent the development of hepatic steatosis in rats( Reference Nagao, Inoue and Wang 5 , Reference Andreoli, Scalerandi and Borel 6 ), whereas a lipoatrophic effect, characterised by a severe reduction of adipose tissue, elevated fat accretion in the liver and hepatomegaly has been described in mice( Reference Poirier, Niot and Clement 7 ). The differential CLA response on TAG metabolism depends on the type of isomers, the level of CLA and fat in the diets and species( Reference Park and Pariza 8 ), among other factors.
The relationship between the intra-uterine environment and the propensity to lipid metabolic alterations in adult life was first noted in response to maternal food restriction( Reference Bursztyn and Ariel 9 ). Similarly, maternal high-fat diet consumption was associated with metabolic disorders including obesity( Reference Li, Yang and Zhu 10 ), hepatic steatosis( Reference Oliveira, Souza and Souza 11 ), glucose intolerance and inflammation in later life( Reference Yokomizo, Inoguchi and Sonoda 12 ). Even though the type and amount of the maternal diet is very important, maternal body fat accumulation during early pregnancy plays a determining role in fetus development and propension or prevention of metabolic alterations in adult life( Reference Herrera, López-Soldado and Limones 13 ). There is limited information about the effects of compounds or agents, such as CLA, that by regulating body fat accretion could modulate the lipid metabolism in offspring animals. In this sense, maternal CLA supplementation in rats fed high-fat diets reverted some negative effects on inflammation, glucose intolerance, insulin insensitivity, hyperlipidaemia and other associated metabolic disorders( Reference Reynolds, Segovia and Zhang 14 – Reference Pileggi, Segovia and Markworth 16 ) in offspring. Moreover, we have previously shown( Reference Veaute, Andreoli and Racca 17 ) that mice fed high-fat diets increased fetal resorption, decreased the IL-4 placental gene expression in the first generation and increased the TNFα gene expression in the second generation. In this generation, CLA normalised Tnfα decreased Il4 and transforming growth factor β1. Nevertheless, at least to the best of our knowledge, there is no information on the metabolic effects of CLA at recommended fat levels. Therefore, the aim of this study was to investigate the effect of maternal CLA consumption on TAG regulation and some inflammatory parameters related with TAG accretion in adult male rat offspring receiving or not receiving CLA.
Methods
Materials and diets
Nutrients and other chemical compounds, vitamins and minerals for the preparation of the diets, were of chemical grade or better, with the exception of soyabean oil (Cada Día), sucrose, cellulose and maize starch, which were obtained from local sources. CLA-mix oil was obtained from Lipid Nutrition B.V. and consisted of an equimolecular mixture of c9t11-CLA and t10c12-CLA. All solvents and reagents used for the fatty acid (FA) quantification were of chromatography grade purchased from Merck. Internal standard glyceryl tritridecanoate (13 : 0-TAG) and external standards GLC-463 containing fifty-two fatty acids methyl esters (FAME) mixture (purity >99 %) were purchased from Nu-Chek. CLA, cis/trans mix (catalogue no. 05507), was purchased from Sigma. Other FAME standards were provided by the International CYTED Net (208RT0343). Primers used for PCR analysis were synthesised by Invitrogen. For the enzyme assays, the materials were from Sigma. All the other chemicals and reagents used were at least American Chemical Society degree or molecular grade and purchased from Merck, Invitrogen and Applied Biosystems. The TAG test kit was obtained from the Sociedad de Bioquímicos. For all the experiments, two isoenergetic diets (Table 1) containing 7 % (w/w) of different dietary fats were used. The control (C) diet was based on the American Institute of Nutrition Ad-Hoc Committee recommendation (AIN-93G) formulated for growth, pregnancy and lactation phases of rodents( Reference Reeves, Nielsen and Fahey 18 ) and contained 7 % of soyabean oil as source of fats. The CLA diet was identical to the C diet, with the exception that 1 % (w/w) of the CLA-mix oil replaced 1 % (w/w) of the soyabean oil. The FA composition of CLA-mix oil (of total FAME) was as follows: 16 : 0, 5·85 %; 18 : 0, 1·20 %; c9-18 : 1, 9·05 %; c11-18 : 1, 0·41 %, c9t11-CLA, 38·99 %; t10c12-CLA, 38·76 %, c9,c12–18 : 2, 1·08 %; and other FA, 4·66 %. The FA composition of diets (Table 2) was determined by GC as indicated below. The diets were freshly prepared every 3 d, gassed with N and stored at 0–4°C.
Table 1 Composition of the experimental diets
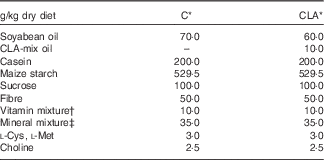
C, control diet; CLA, conjugated linoleic acid diet.
* Homemade prepared based on the American Institute of Nutrition Ad-Hoc Committee recommendation (AIN-93G) formulated for growth, pregnancy and lactation phases of rodents( Reference Reeves, Nielsen and Fahey 18 ).
† Vitamin mixture ( per kg of diet): nicotinic acid, 30·0 mg; pantothenate, 15·0 mg; pyridoxine, 6·0 mg; thiamin, 5·0 mg; riboflavin, 6·0 mg; folic acid, 2·0 mg; vitamin K, 750·0 μg; d-biotin, 200·0 μg; vitamin B12, 24·0 μg; retinyl acetate, 1·2 mg; cholecalciferol, 0·025 mg; DL-α-tocopheryl acetate, 50 mg.
‡ Mineral mixture (mg/kg of diet): Ca, 5000·0; P, 1561·0; K, 3600·0; S, 300·0; Na 1019·0; Cl, 1571·0; Mg, 507·0; Fe, 35·0; Zn, 30·0; Mn, 10·0; Cu, 6·0; iodine, 0·2; Mo 0·15; Se, 0·15; Si, 5·0; Cr 1·0; F, 1·0; Ni, 0·5; B, 0·5; Li, 0·1; and V, 0·1.
Table 2 Fatty acid composition of experimental diets
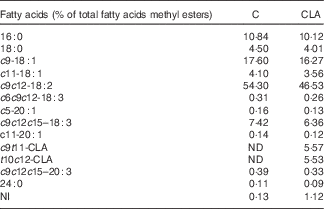
C, control diet; CLA, conjugated linoleic acid diet; ND, not detected; NI, not identified.
Animals and experimental design
All the experiments were conducted in compliance with the regulations of the School of Biochemistry (Universidad Nacional del Litoral) and Guide to the Care and Use of Experimental Animals of Laboratory( Reference Bayne 19 ). Male and virgin mature female Wistar rats were provided from the facilities at our University. The animals were housed in collective cages under controlled conditions (23±2 °C and 12 h light–12 h dark) and free access to standard food and water. After 1 week of adaptation period, the female rats were randomly divided into two groups (n 6/group) and fed C or CLA diets (initial weights: 274·1 (sem 8·6) and 269·9 (sem 5·7) g, respectively) for 4 weeks before mating and throughout pregnancy and lactation. At parturition time, litter size was standardised to eight pups per dam in both C- and CLA-fed mothers. The pups were maintained with their own mothers until weaning. In all, twelve post-weaning male pups were randomly selected from C-fed mothers and received the same diet (C/C group). A total of twenty-four post-weaning male pups from CLA-fed mothers were randomly divided into two subgroups. One subgroup of twelve pups was fed the CLA diet (CLA/CLA group) and the other subgroup of twelve pups was fed the C diet (CLA/C group). The animals from the C/C, CLA/C and CLA/CLA groups were fed the respective diets for 9 weeks.
After the dietary treatment, one set of 6 animals per group was fasted overnight and killed (09.00–11.00 hours) under anaesthesia (1 mg azepromazine+100 mg ketamine/kg body weight) by cardiac exsanguination. Blood was collected and serum was obtained after centrifugation (1000 g for 10 min at 4°C). Liver, gastrocnemius muscle (GM) and epididymal white adipose tissue (EWAT) were dissected, weighed and immediately frozen. All samples were stored at −80°C until analysis.
Fatty acid composition in liver, epididymal white adipose tissue, serum and dietary lipids
The CLA levels in liver, EWAT and serum, and the FA composition of experimental diets, were determined by GC using a Shimadzu (GC 2014) chromatograph equipped with a flame ionisation detector. Analyses were carried out with a capillary column CP Sil 88 (100 m, 0·25 µm film thickness; Varian). The carrier gas was H2 with a split ratio of 1:10. The column temperature was held at 75°C for 2 min after injection, and then 5°C/min to 170°C, held for 40 min, 5°C/min to 220°C and held for 40 min. Injection volume was 0·5 µl and the column flow was 0·8 ml/min. Total fat in tissues, serum and diets was extracted using the method described by Bligh & Dyer( Reference Bligh and Dyer 20 ). The FAME were formed by transesterification with methanolic potassium hydroxide solution as an interim stage before saponification (ISO 5509 : 2000, Point 5 International Union of Pure and Applied Biochemistry method 2.301). The FAME were identified by comparison of their retention times relative to those of commercial standards. Values of FA content were expressed as percentage of total FA. Further GC details could be obtained from previous publications( Reference Saín, Gonzalez and Lavandera 21 , Reference Fariña, González and Scalerandi 22 ).
TAG levels in serum and liver
TAG levels in serum were determined by spectrophotometric methods using a commercially available test kit. Portions of frozen tissue were powdered and homogenised in saline solution (10 %, w/v) for TAG determination( Reference Laurell 23 ).
Lipoprotein lipase activity in epididymal white adipose tissue and gastrocnemius muscle
The adipose tissue lipoprotein lipase (LPL) enzyme activity was quantified in EWAT acetone powder by the fluorometric method of Del Prado( Reference Del Prado, Hernandez-Montes and Villalpando 24 ). To assess muscle LPL activity, GM samples were homogenised in a NH4Cl/NH4OH-Heparin buffer and the LPL activity quantification was performed by the technique of Del Prado( Reference Del Prado, Hernandez-Montes and Villalpando 24 ). Values were expressed as nmol fluorescein/min per total tissue or as nmol fluorescein/min per g tissue. Further methodological details about the assessment have been previously published( Reference Fariña, González and Scalerandi 22 ).
Lipogenic and β-oxidative enzyme activities
In liver homogenates, acetyl-CoA carboxylase (ACC; EC 6.4.1.2), fatty acid synthase (FAS; EC 2.3.1.85) and glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) activities, as lipogenic enzymes, were measured according to the methods of Zimmerman( Reference Zimmerman 25 ), Lynen( Reference Lynen 26 ) and Kuby & Noltmann( Reference Kuby and Noltmann 27 ), respectively. The enzyme activities were expressed either as mU/mg of protein, where 1 mU was 1 nmol NADH consumed (ACC), 1 nmol NADPH consumed (FAS) or 1 nmol NADPH produced (G6PDH) per min. Carnitine palmitoyltransferase Ia (CPT-Ia; EC 1.3.99.3) activity, as β-oxidative enzyme, was assessed in the liver mitochondrial fraction by the method described by Bieber et al.( Reference Bieber, Abraham and Helmrath 28 ) and expressed as mU/mg of protein (1mU=1 nmol CoA/min). Protein content was determined using bovine serum albumin as standard by the Lowry method( Reference Lowry, Rosebrough and Farr 29 ).
Hepatic mRNA levels of lipogenic and β-oxidative enzymes, transcriptional factors and inflammatory cytokines
Total RNA was isolated from liver using Trizol according to the manufacturer’s instructions. RNA samples were then treated with a DNA-free kit (Applied Biosystems) to remove any contamination with genomic DNA. The yield and quality of the RNA were assessed by measuring absorbance at 260, 270, 280 and 310 nm and by electrophoresis on 1·3 % agarose gels. A measure of 1·0 µg of total RNA of each sample was reverse-transcribed to first-strand complementary DNA (cDNA) using a M-MLV RT. Relative mRNA levels were quantified using real-time PCR with a StepOne 18 TM Real-Time PCR Detection System (Applied Biosystems). Sequence-specific primers were used (Genbank: ACC, NM_022193.1; FAS, NM_017332.1; sterol regulatory element-binding protein-1c (SREBP-1c), NM_001276708.1; stearoyl-CoA desaturase-1a (SCD-1a), NM_139192.2; CPT-Ia, NM_031559.2; PPARα, NM_013196.1; IL-6, NM_012589.2; IL-1β, NM_031512.2; PPARγ, NM_013124.3; β–actin, NM_031144.3; ubiquitin C (UBC), NM_017314.1 and hypoxanthine phosphoribosyltransferase 1 (HPRT1), NM_012583.2), commercially synthesised (Invitrogen Custom Primers) and the sequences were as follows: ACC: 5'-AAC AGT GTA CAG CAT CGC CA-3' (forward), 5'-CAT GCC GTA GTAG GTT GAG GT-3' (reverse); FAS, 5'-CAG AAC TCT TCC AGG ATAG TCA ACA -3' (forward), 5'- GTC GCC CTAG TCA AGG TTC AG -3' (reverse); SREBP-1c, 5'- GGA GCC ATAG GAT TAGC ACA TT-3' (forward), 5'- GCT TCC AGA GAG GAG CCC AG-3' (reverse); SCD-1, 5'- CAC ACG CCG ACC CTC ACA ACT-3' (forward), 5'-TCC GCC CTT CTC TTT GAC AGC C-3' (reverse); CPT1, 5'- ACG TAGA GTAG ACT GGT GGG AAG AAT-3' (forward), 5'-TCT CCA TAGG CGT AGT AGT TAGC TAGT-3' (reverse); PPARα, 5'- CCC CAC TTAG AAG CAG ATAG ACC-3' (forward), 5'- CCC TAA GTA CTAG GTA GTC CGC-3' (reverse); IL-6, 5'- CCT TCT TAGG GAC TAGA TAGT TAGT TAGA C-3' (forward), 5'- GGG TAGG TAT CCT CTAG TAGA AGT CTC C-3' (reverse); IL-1β, 5'-AGG CAG TAGT CAC TCA TTAG TAGG C-3' (forward), 5'- TCA CAT GGG TCA GAC AGC ACG-3' (reverse); PPARγ, 5'- CGG AGT CCT CCC AGC TAGT TCG CC-3' (forward), 5'- GGC TCA TAT CTAG TCT CCG TCT TC-3' (reverse); β–actin 5'- CAT GAA GAT CAA GAT CAT TAGC TCC T-3' (forward), 5'-CTAG CTT GCT GAT CCA CAT CTAG-3' (reverse); UBC 5'- ACACCAAGAAGGTCAAACAGGA-3' (forward), 5'-CACCTCCCCATCAAACCCAA-3' (reverse); HPRT1 5'-TCCTCCTCAGACCGCTT TTC-3' (forward), 5'-ATCACTAATCACGACGCTGGG-3' (reverse). Standard curves for each primer were generated on separate runs using several serial dilutions (1/10–1/1000) of pooled cDNA samples. The corresponding primer efficiency (E) of one cycle in the exponential phase was calculated according to equation E=10(−1/slope)
(
Reference Rasmussen
30
). All the efficiencies of the primers were 100 (sem 10) %. Target genes were normalised with the geometric mean of three housekeeping genes: β-actin, UBC and HPRT1(
Reference Vandesompele, De Preter and Pattyn
31
). Relative expression ratios were calculated using the recommended
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak, Schmit and Gen
32
).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak, Schmit and Gen
32
).
Hepatic TAG secretion rate
Another set of six animals per group was used; the animals were fasted overnight and anaesthetised as mentioned above. Then, 600 mg/kg body weight of triton WR 1339 in saline solution, an agent known to inhibit the peripheral removal of TAG-rich lipoproteins, was injected intravenously( Reference Otway and Robinson 33 ). Blood samples were taken immediately before and 120 min after the injection of the triton solution for the estimation of TAG accumulation in serum. Hepatic TAG secretion rate (TAG-SR) was estimated based on serum TAG concentration at 0 and 120 min, plasma volume and body weight. Further details have been previously reported( Reference Andreoli, Gonzalez and Martinelli 34 ).
Statistical analysis
Values were expressed as means with their standard errors of six animals per group. The minimum sample size needed to detect a statistically significant difference (P<0·05) was calculated( Reference Bowker and Lieberman 35 ). A sample size of six had an 80 % power (P=0·05). The statistical analysis was performed by one-way ANOVA (1×3) followed by Scheffe’s test (P<0·05), using SPSS version 17.0 (SPSS Inc.), except for the comparison between CLA isomers shown in Table 4 for which Student’s t test was used. All post hoc multiple comparisons were made using Tukey’s critical range test. Significant differences were considered at P<0·05.
Results
Nutritional parameters and TAG content in serum and liver
During all the experiment in dams and offspring, the diets were well accepted and the animals showed a healthy status without any pathological manifestation owing to the treatments received. The body weights (g) of dams after 4 weeks of dietary treatment before mating were C: 357·2 (sem 14·3) and CLA: 347·4 (sem 15·7) and the mean food intakes (kJ/d) were C: 255·9 (sem 12·0) and CLA: 253·3 (sem 5·8). Body and tissue weights of offspring and serum and liver TAG content are shown in Table 3. The final body weights decreased in groups CLA/CLA and CLA/C, parallel to a significant decrease in the EWAT weights. The absolute liver weight was lower in the CLA/CLA and CLA/C groups, but their relative weights were not affected by the dietary treatments. Serum TAG levels were increased in the CLA/CLA and CLA/C groups, and liver TAG levels were decreased in the CLA/CLA group, but not in the CLA/C group.
Table 3 Body and tissue weights and serum and liver TAG content of offspring (Mean values with their standard errors; n 6 animals/group)
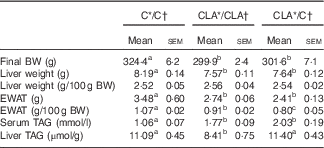
C, control diet; CLA, conjugated linoleic acid diet; BW, body weight; EWAT, epididymal white adipose tissue.
a,b,c Mean values in the same row with unlike superscript letters were significantly different (P<0·05).
Statistical analyses between different groups were established by one-way ANOVA (1×3), followed by Scheffe’s test.
* Diet fed to the dam.
† Diet fed to the male offspring.
Percentage of individual conjugated linoleic acid in serum, liver and epididymal white adipose tissue
The percentage of individual CLA isomers in serum and tissues of offsprings was expressed as % of total FAME (Table 4). Both CLA isomers were incorporated in serum, liver and EWAT of the CLA/CLA group, but we did not detect any CLA isomer in CLA/C rats. The percentage of individual CLA in the tissues of CLA/CLA rats was EWAT > liver > serum. In addition, all tissues showed higher levels of c9t11-CLA than the t10c12-CLA isomer.
Table 4 Percentage of individual conjugated linoleic acid (CLA) (% of total fatty acids methyl esters) in serum, liver and epididymal white adipose tissue (EWAT) of offspring (Mean values with their standard errors; n 6 animals/group)
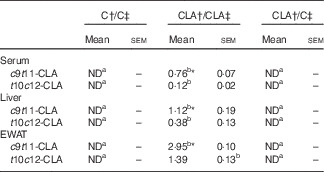
C, control diet; ND, not detected.
a,b Mean values in the same row with unlike superscript letters were significantly different (P<0·05).
Statistical analyses between different groups were established by one-way ANOVA (1×3), followed by Scheffe’s test.
*Statistical differences between c9t11-CLA and t10c12-CLA isomers were established by Student’s t test (P<0·05).
† Diet fed to the dam.
‡ Diet fed to the male offspring.
Serum and liver TAG regulation
The serum fasting TAG levels were mainly modulated by liver TAG secretion and peripheral TAG removal (Table 5). The hepatic TAG-SR did not change in the CLA/CLA group; however, it was significantly decreased in the CLA/C group. The serum TAG removal rate was estimated by the EWAT and GM LPL activities. The EWAT LPL activity, expressed either by total tissue or by g of tissue, was diminished in the CLA/CLA and CLA/C groups; however, this activity was not modified by any dietary treatment in the GM.
Table 5 Serum and liver TAG regulation of offspring (Mean values with their standard errors; n 6 animals/group)
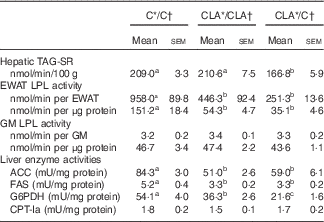
C, control diet; CLA, conjugated linoleic acid diet; TAG-SR, TAG secretion rate; EWAT, epididymal white adipose tissue; GM, gastrocnemius muscle; LPL, lipoprotein lipase; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; G6PDH, glucose-6-phosphate dehydrogenase; CPT-Ia, carnitine palmitoyltranferase-Ia; 1 mU, 1 nmol NADH consumed/min (ACC); 1 nmol NADPH consumed/min (FAS); 1 nmol/NADPH produced per min (G6PDH); 1 nmol CoA/min (CPT-Ia).
a,b,c Mean values in the same row with unlike superscript letters were significantly different (P<0·05). Statistical analyses between different groups were established by one-way ANOVA (1×3), followed by Scheffe’s test.
* Diet fed to the dam.
† Diet fed to the male offspring.
The fasting hepatic TAG levels were mostly regulated by lipogenesis, β-oxidation and hepatic TAG-SR (Table 5). The ACC, FAS and G6PDH activities were diminished in the CLA/CLA and CLA/C groups. Meanwhile, the activity of the main enzyme involved in the hepatic FA oxidation, CPT-Ia, was not modified in the CLA/CLA and CLA/C groups.
The liver expression of some key genes and transcriptional factors related to FA biosynthesis (Acc, Fas, Scd1 and Srebpf1c) and β-oxidation (Cpt1a, Ppara), as well as inflammation (Il6, Il1b, Pparg), are shown in Table 6. The levels of mRNA of ACC, FAS, SCD-1a and SREBP-1c were diminished in the CLA/CLA group. The ACC, but not FAS, SCD-1 and SREBP-1c, gene expression was decreased in the CLA/C group. As regards fatty acid oxidation, CPT-Ia and PPARα mRNA levels were unchanged in CLA/CLA group, but decreased in the CLA/C group. In addition, some of the genes related to inflammation were assayed, showing a significant decrease in the Pparg and Il1b expression in both CLA/CLA and CLA/C. No changes were observed in IL-6 mRNA levels.
Table 6 Hepatic mRNA levels of lipogenic and β-oxidative enzymes, transcriptional factors and inflammatory cytokines of offspring (Mean values with their standard errors; n 6 animals/group (relative units))
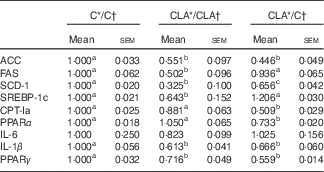
C, control diet; CLA, conjugated linoleic acid diet; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; SCD-1, stearoyl-CoA desaturase-1; SREBP-1c, sterol regulatory element-binding protein 1c, CPT-Ia, carnitine palmitoyltransferase Ia.
a,b Mean values in the same row with unlike superscript letters were significantly different (P<0·05). Statistical analyses between different groups were established by one-way ANOVA (1×3), followed by Scheffe’s test.
* Diet fed to the dam.
† Diet fed to the male offspring.
Discussion
Dietary CLA reduces body fat accumulation and modulates lipid metabolism, as well as the inflammatory response, which could have a potential key role during the gestation period. The CLA-mix oil contains mainly c9t11-CLA and t10c12-CLA in equimolecular proportion; however, their biological effects are different. Even though both isomers are involved in lipid metabolism regulation, t10c12-CLA is principally responsible for increasing lipolysis and the β-oxidation/lipogenesis ratio modulating fat accretion( Reference Poirier, Niot and Clement 7 ), whereas the c9t11-CLA has an anti-inflammatory response( Reference Wang, Liu and Zhang 36 ). Taking into account that the reduction in lipid depots might induce metabolic changes in the offspring, increasing the risk of metabolic alteration in adult life( Reference Herrera, López-Soldado and Limones 13 ), the aim of this study was to investigate the effect of maternal CLA consumption on nutritional parameters, TAG levels in serum and liver, as well as their regulation in adult offspring rats that received CLA or not. In addition, some gene expression factors related to the inflammatory process were studied. To the best of our knowledge, this is the first study to investigate the influence of maternal CLA on TAG metabolism in adult offspring rats fed normo-energetic diets.
It is known that CLA reduces the fat mass by different mechanisms including decreased fatty acid uptake, increased fatty acid oxidation, increased overall energy expenditure and others( Reference Pariza, Park and Cook 1 ). In agreement with several studies in different experimental animal models fed CLA( Reference Park and Pariza 8 , Reference Andreoli, Gonzalez and Martinelli 34 , Reference Ryder, Portocarrero and Song 37 ), the present results in offspring male rats fed CLA (CLA/CLA group) showed a lower final body weight related to reduced fat depots. It is important to note that the results on rat body composition are controversial, depending on the type of isomer and level of CLA, nutritional status and time of feeding( Reference Bhattacharya, Banu and Rahman 3 , Reference Park and Pariza 8 ). There are few studies showing the effect of CLA on adult offspring rats fed the same diet as the dams. In a previous study from our laboratory( Reference Veaute, Andreoli and Racca 17 ), CLA reduced the EWAT weights at first and second generation of mice, and similar results have been obtained in rats of the first generation( Reference Reynolds, Segovia and Zhang 14 ). In the present study, the effects on fat depots were observed in the presence of high CLA levels in adipose tissue (CLA/CLA group) and in the absence of CLA (CLA/C group). It is well known that CLA isomers are incorporated and metabolised into the tissues, and the rate of CLA metabolism is sensitive to experimental conditions( Reference Banni 38 ). However, to the best of our knowledge, there is no study showing the disappearance rate of CLA in the offspring of CLA-fed mothers; therefore, the measurement of CLA levels in tissues of the CLA/C group was performed to warrant the absence of CLA in tissues of offspring. Thus, the metabolic effects of the diets in the offspring could be specifically attributed to maternal CLA, which was administered to the dams during pregnancy and lactation. Interestingly, those adult offspring from CLA-fed rats that were fed C diets after weaning (CLA/C group) also showed a reduction of body and EWAT weights. It is noteworthy that in both offspring from CLA dams the reduction of EWAT was associated with a lower LPL enzyme activity. These results might hypothesise that a programming mechanism of CLA during early life could be involved in controlling the adiposity in offspring through the fatty acid uptake by the EWAT.
The plasma TAG levels were regulated by the circulating TAG removal rate from the adipose tissue and muscle, as well as by the hepatic VLDL-TAG secretion. As the hepatic VLDL-TAG-SR was not increased and the muscle LPL enzyme activity did not change by the dietary treatments, the reduced LPL activity in EWAT could explain the higher plasma TAG levels in both CLA/C and CLA/CLA groups. A similar association between the increase of plasma TAG and the reduction of adipose tissue LPL activity was found in mice fed different types of oils supplemented with 1 % CLA-mix oil at normal fat levels( Reference Scalerandi, Gonzalez and Saín 39 ) and hamsters fed high-fat diets supplemented with a similar commercial CLA( Reference Deckere, van Amelsvoort and McNeill 40 ). However, compared with results previously observed in mice( Reference Scalerandi, Gonzalez and Saín 39 ), in the present work, the VLDL-TAG-SR did not contribute to the hypertriacylglycerolaemia. Other authors found a reduction in mice fed high-fat diets( Reference Andreoli, Gonzalez and Martinelli 34 , Reference Liu, Purushotham and Wendel 41 ) or no changes in serum TAG in rats fed diets rich in saturated fats( Reference Kloss, Linscheid and Johnson 42 ). Thus, the differences observed in the effect of CLA-mix oil are not only owing to the species but also to the type of isomer and dietary experimental protocol, among other reasons.
Liver is an organ that plays a crucial role in lipid metabolism and has been reported to be a key target for CLA( Reference Pariza, Park and Cook 1 ). As shown in EWAT, CLA was incorporated into the liver of CLA/CLA, but was not detected in the CLA/C group. In the CLA/CLA group, the percentage of c9t11-CLA was higher than that of the t10,c12-CLA isomer. Similar results have been previously shown in animals fed CLA diets( Reference Purushotham, Shrode and Wendel 43 ). The effect of CLA on hepatic TAG has been proposed to be species specific( Reference Vyas, Kadegowda and Erdman 44 ). In contrast to the effects described by CLA inducing a significant liver TAG accretion in mice( Reference Poirier, Niot and Clement 7 , Reference Andreoli, Gonzalez and Martinelli 34 ), neither liver steatosis nor hepatomegaly was observed in both groups of offspring rats from CLA-fed dams. Furthermore, CLA/CLA rats showed a reduction of hepatic TAG content. The lowering effect of CLA on hepatic TAG has been previously shown in rats under different conditions of liver steatosis ( Reference Purushotham, Shrode and Wendel 43 , Reference Andreoli, Illesca and González 45 – Reference Wang, Nagao and Inoue 49 ). Purushotham et al.( Reference Purushotham, Shrode and Wendel 43 ) proposed that CLA attenuated hepatic steatosis in adult Wistar rats by modulating hepatic genes involved in lipogenesis and lipid oxidation. In addition, these authors suggested that the dissimilar effects of CLA on hepatic steatosis and insulin sensitivity may depend on differences in fat-to-lean partitioning between adipose and liver tissues in numerous animal models. Interestingly, in both groups of rats from CLA-fed dams, the lipogenesis was significantly reduced. Specifically, in the CLA/CLA group, the lower ACC, FAS and G6PDH activities and reduced mRNA levels of ACC, FAS, SCD-1 and SREBP-1c, with normal CPT-Ia activity and mRNA levels clearly demonstrated an unbalance between lipogenesis and β-oxidation. In agreement with our results, CLA decreased the hepatic FAS( Reference Azain, Hausman and Sisk 50 ) and SCD-1( Reference Choi, Park and Pariza 51 ) activities and FAS( Reference Maslak, Buczek and Szumny 52 ) and SCD-1( Reference Choi, Park and Pariza 51 , Reference Maslak, Buczek and Szumny 52 ) mRNA levels. In addition, an unchanged FA oxidation has also been observed( Reference Park, Albright and Liu 53 ). However, some reports have shown increased( Reference Ide 54 , Reference Degrace, Demizieux and Gresti 55 ) and reduced( Reference Rasooly, Kelley and Greg 56 ) FA oxidation. A reduced lipogenesis was also observed in the CLA/C group, but in contrast to the CLA/CLA group two different compensatory mechanisms might be involved to balance the hepatic TAG levels. Specifically, a decreased β-oxidation, as a result of a lower CPT-1a gene expression, and a reduction of hepatic TAG secretion contributed to maintain the normal hepatic TAG content. The most interesting and original result is that a lower ACC, FAS and G6PDH enzyme activity and a diminished mRNA level of ACC was also observed even in the absence of CLA in the diet. Reinforcing the above-mentioned effects observed in body composition, CLA might programme the lipid metabolism driving to a prevention of liver TAG accretion. There are no studies reporting data to contrast with the present findings.
The beneficial effects of CLA on immune and inflammatory responses in different animal models, describing the mechanism of action of CLA, particularly the c9,t11–18 : 2, have recently been reviewed( Reference Viladomiu, Hontecillas and Bassaganya-Riera 57 ). Thus, after CLA exposure, a reduction of pro-inflammatory cytokine production has been described, mainly IL-6, TNFα, IFNγ, and IL-1β, which could play an important role in the pathogenesis of many chronic-inflammation-mediated diseases( Reference O’Shea, Bassaganya-Riera and Mohede 58 , Reference Bassaganya-Riera and Hontecillas 59 ). Reynolds et al.( Reference Reynolds, Segovia and Zhang 14 ) and Segovia et al.( Reference Segovia, Vickers and Zhang 15 ) demonstrated in different experimental animal models that CLA reversed maternal metainflammation induced by high-fat diet and prevented programming of adverse offspring metabolic outcomes. Our results also show that CLA might down-regulate the liver gene expression of some pro-inflammatory factors such as IL-1β and PPARγ in offspring, even at recommended dietary fat levels.
In brief, these results demonstrate a programming effect of CLA on lipid metabolic pathways leading to a preventive effect on the TAG accretion in adipose tissue and liver of male rat offspring. This knowledge could be important to develop some dietary strategies to reduce the incidence of obesity and fatty acid liver disease in humans.
Acknowledgements
The authors wish to thank Walter DaRú for his technical assistance.
This study was supported by Universidad Nacional del Litoral-Cursos de Acción para la Investigación y el Desarrollo (CAI+D 2011 nos 501 201101 00165 LI and 501201101 00166 LI)-Secretaría de Ciencia y Técnica-UNL and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP CONICET no. 112-200801-00786).
C. A. B. designed and planned the study, as well as analysed results, interpreted findings and wrote the manuscript; J. V. L. conducted the study, performed the sample analysis, analysed the data, interpreted findings and prepared the manuscript; C. D. G., J. S. and A. C. F. carried out collections and analytical determinations and assisted in the maintenance and killing of rats; M. A. G. contributed to the design and planning of the study, interpreted findings and discussed the manuscript.











