Article contents
Chemically controlled surface compositions of Ag–Pt octahedral catalysts
Published online by Cambridge University Press: 23 March 2017
Abstract
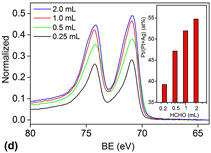
A hydrothermal method was developed to synthesize Ag–Pt nanoparticles with controlled surface composition where formaldehyde (HCHO) was utilized as a directing agent. Transmission electron microscopy and powder x-ray diffraction characterizations showed no change in bulk composition and phases as well as the size and morphology of as-made bimetallic nanocrystals. X-ray photoelectron spectroscopy study revealed, however, the enrichment of Pt on the surface as the amount of HCHO used increased. This chemically driven change in surface composition represents a nontraditional approach in the control of synthesis of bimetallic nanoparticle catalysts. A close relationship between catalytic performance and surface composition of these Ag–Pt nanocrystals was observed for electrochemical oxidation of formic acid.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 1
- Cited by





