Article contents
Preparation and photocatalytic performance of Ag/AgCl-modified cubic ZHS hollow particles
Published online by Cambridge University Press: 28 May 2014
Abstract
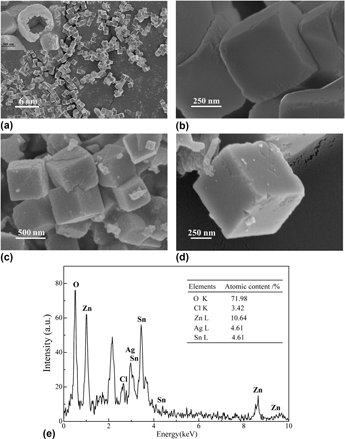
SnZn(OH)6 (ZHS) hollow cubes were synthesized by a facile self-templated method at room temperature, and Ag/AgCl/ZHS particles were prepared by a photodepositing method. The crystalline structure and morphology of the prepared particles were characterized by x-ray diffraction, UV-vis diffuse reflectance spectroscopy, scanning electron microscopy, and N2 adsorption. The results indicated that the particles had almost uniform monoclinic geometry and size. The photocatalytic oxidation of Rhodamine B was used to evaluate the photocatalytic activity of the synthesized photocatalysts. It is found that the hollow Ag/AgCl/ZHS showed the highest catalytic performance under visible or UV light, which can be attributed to the synergetic effect of Ag, AgCl, and ZHS.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 4
- Cited by




