The Dietary Reference Intakes Committee in Taiwan (Wei et al. Reference Wei, Chang and Huang2003) and in the USA (Institute of Medicine, 1998) extrapolated the vitamin B6 estimated average requirement and RDA for children and adolescents aged 1–18 years from adult values due to limited information. We found that levels of plasma pyridoxal-5′-phosphate (PLP) and total aldehyde B6 in 7–12 and 13–18-year-old groups were the lowest in all age groups studied in the first Nutrition and Health Survey in Taiwan 1993–1996 (NAHSIT) (Chang et al. Reference Chang, Fan, Yeh and Pan1999). Unfortunately, vitamin B6 intakes were not analysed due to the lack of dietary information in NAHSIT. Therefore, these age groups were further studied by our laboratory concerning vitamin B6 requirements because the status of this nutrient is possibly marginal among various population groups. We have reported the vitamin B6 requirement for children aged 7–12 years (Chang et al. Reference Chang, Huang, Hsiao, Lee and Hsuen2002) and adolescents aged 13–15 years (Chang et al. Reference Chang, Hsiao and Hsuen2003). The dietary intake, nutritional status and functional consequences of a certain range of intake are suggested in determining nutrient requirements (King, Reference King1996). In the present study, vitamin B6 status and its relationship to dietary intake of high school students was studied to investigate the requirement. We evaluated the effect of vitamin B6 intake on adequate vitamin B6 status indicators in plasma, erythrocytes and urine of high school students determined to be healthy by anthropometric measurements. The median intake of vitamin B6 was determined from the adequate levels of all status indicators and the requirement was suggested.
Materials and methods
Subjects
Healthy senior high school students (eighty-three boys and seventy-four girls) aged 16–18 years from two out of nineteen high schools in Tainan, the southern part of Taiwan, were randomly recruited in the present study. The experimental procedures were reviewed and approved by the Review Board of the Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan, and explained to the students and parents before submission of the consent form. Informed consent was obtained from both the students and their parents. Students who were not in good health, had any diseases interfering with vitamin B6 metabolism or were taking medicines and/or supplements altering vitamin B6 status were not included in this study. A total of 157 students participated and 127 (sixty-five boys and sixty-two girls) completed the study with both anthropometric and biochemical data. Those who withdrew from the study only had anthropometric data. All measurements, including dietary intake, anthropometry and vitamin B6 status, were made at the beginning and end of the semester and averaged to determine the data for each student at the age studied.
Dietary intake assessment
Dietary records (3 d; one record was for a weekend day and the other two for weekdays) were obtained with a follow-up by personal interview to minimize uncertainties (Reynolds, Reference Reynolds1990). A trained dietary interviewer assisted the students in using the chart of food portion sizes provided by the Department of Health, Executive Yuan, Taiwan. A computer program, Nutritionist IV (N-squared, Salem, OR, USA), was used and foods unique to Taiwan were added into the program to calculate dietary energy and nutrient intakes.
Anthropometric measurements
Anthropometric measurements including height, weight, midarm circumference, tricep skin-fold thickness and percentage body fat, were made by a trained interviewer. Body weight and height were measured with subjects wearing school uniforms but without shoes. Body weight and percentage body fat were measured simultaneously using a body fat monitor/scale (TBF-531; Tanita, Tokyo, Japan) Weight and height measurements were used to calculate BMI (kg/m2). Midarm muscle circumference was calculated from the midarm circumference. Tricep skin-fold thickness was measured by skin-fold calipers (Lafayette Instrument, Lafayette, IN, USA). Anthropometric data were compared with that of the NAHSIT 1993–1996 (Kao et al. Reference Kao, Tzeng, Yeh, Cheng and Pan1999) for the appropriate age group.
Sample collection
Venous blood was collected from fasting students in vacutainer tubes containing EDTA between 0800 and 0900 hours on the second day of the 3-d dietary record, kept in crushed ice and protected from light. Blood samples were centrifuged at 3000 g and 4°C for 10 min. Plasma was removed and aliquots were frozen at − 40°C for plasma PLP analyses. Erythrocytes were washed three times with saline and an aliquot of packed cells was removed for assay of erythrocyte alanine activity coefficient (EALT-AC) and aspartate aminotransferase activity coefficient (EAST-AC).
A 24-h urine collection was obtained on the same day of blood sample collection using toluene as preservative. Aliquots of urine were stored at − 40°C for urinary 4-pyridoxic acid (4-PA) analyses.
Laboratory analysis
Plasma PLP concentrations were determined by HPLC with fluorometric detection (Kimura et al. Reference Kimura, Kanehira and Yokoi1996). The recovery of added PLP from plasma was 102·3 (sd 3·3) %. Within- and between-day reproducibilities were 1·46 and 2·46 %, respectively. EALT-AC and EAST-AC were measured with and without added PLP (Woodring & Storvick, Reference Woodring and Storvick1970) on the same day that blood was drawn. The EALT-AC and EAST-AC were calculated as the ratio of simulated (PLP added) to unstimulated (no PLP added) activities. Urinary 4-PA was analysed by HPLC with fluorometric detection (Gregory & Kirk, Reference Gregory and Kirk1979).The recovery of added 4-PA from urine was 90·5 (sd 0·3) % and the 4-PA data were corrected for recovery. Within- and between-day reproducibilities were 4·13 and 4·13 %, respectively.
Statistical analyses
Data were analysed using the SAS statistical analysis computer program (version 6.12; SAS Institute, Cary, NC, USA) and expressed as means and standard deviations unless otherwise stated. The general linear model was performed to determine the differences between group means at the beginning and end of the semester and between boys and girls for daily dietary intakes and vitamin B6 status measures. One-way ANOVA was used to test the differences among the means of vitamin B6 intakes of students who had adequate vitamin B6 status indicators. Pearson correlation coefficients were computed to determine relationships among vitamin B6 status measures and vitamin B6 intakes. The level of significance was considered to be P < 0·05, unless otherwise stated. The percentages of students with adequate plasma PLP, EALT-AC, EAST-AC and urinary 4-PA (Leklem, Reference Leklem1990) were calculated. Median intakes for those who had adequate status of B6 indicators were determined and used for the suggestion of vitamin B6 requirement.
Results
Anthropometric data
Most anthropometric data (Table 1) were similar to those of NAHSIT, 1993–1996 (Kao et al. Reference Kao, Tzeng, Yeh, Cheng and Pan1999), indicating normal growth of the students.
Table 1 Anthropometric measurements of high school boys and girls aged 16–18 years * † (Mean values and standard deviations)

* For details of subjects and procedures, see Materials and methods.
† Values measured at the beginning and end of the semester for each student were averaged and used to calculate the mean.
MAC, midarm circumference; MAMC, midarm muscle circumference TSF, triceps skin-fold thickness.
Dietary intakes
Energy and protein intakes of boys were higher than those of girls, but vitamin B6 intake was similar (Table 2).
Table 2 Daily energy, protein and vitamin B6 intakes and the B6:protein ratios of boys and girls aged 16–18 years† (Mean values and standard deviations)
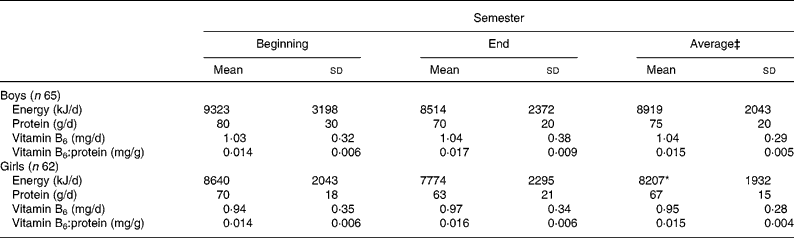
Mean values are significantly different from those of the boys; *P < 0·05.
† For details of subjects and procedures, see Materials and methods.
‡ Average of two values measured at the beginning and end of the semester for each student was used to calculate the mean.
Biochemical status of vitamin B6
Plasma PLP concentration, EALT-AC, EAST-AC and urinary 4-PA excretion measured at the beginning and end of the semester were not significantly different and were averaged to obtain the individual biochemical indicators of vitamin B6 (Table 3).
Table 3 Plasma, urinary and erythrocyte vitamin B6 status measures of boys and girls aged 16–18 years† (Mean values and standard deviations)
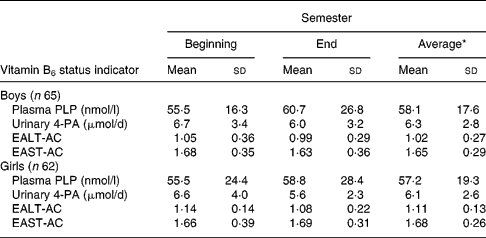
* Average of two values measured at the beginning and end of the semester for each student was used to calculate the mean. No significant differences were detected by general linear model.
† For details of subjects and procedures, see Materials and methods.
EALT-AC, erythrocyte alaline aminotransferase activity coefficient; EAST-AC, erythrocyte aspartate aminotransferase activity coefficient; 4-PA, 4-pyridoxic acid; PLP, pyridoxal phosphate.
Plasma PLP concentrations for all students except one girl (28·7 nmol/l) were >35 nmol/l (Table 4). Adequate plasma PLP concentration >35 nmol/l was used in the present study because a 20 nmol/l of PLP concentration suggested by the dietary reference intakes for adults (Institute of Medicine, 1998) may not reflect normal status for this age group. The percentages of students with adequate vitamin B6 status evaluated by PLP >35 nmol/l EALT-AC < 1·25, EAST-AC < 1·8 and urinary 4-PA excretion >3·0 − μmol/d (Leklem, Reference Leklem1990) were quite different among these four indicators in both boys and girls. However, the vitamin B6 intakes of the students who had adequate values of the different indicators were not different from one another and ranged from 1·04 (sd 0·29) to 1·16 (sd 0·26) mg/d in boys and from 0·96 (sd 0·27) to 1·06 (sd 0·27) mg/d in girls.
Table 4 Percentages and means of vitamin B6 intake and dietary vitamin B6:protein ratio of boys and girls with adequate vitamin B6 status * † (Mean values and standard deviations)
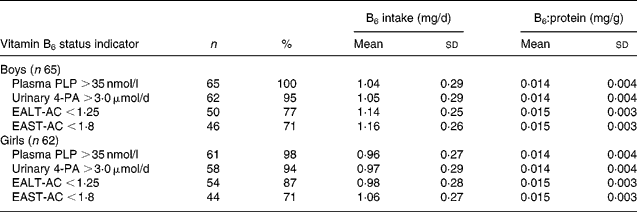
* For details of subjects and procedures, see Materials and methods.
† Based on the average of two values measured at the beginning and end of the semester for each student.
EALT-AC, erythrocyte alanine aminotransferase activity coefficient; EAST-AC, erythrocyte aspartate aminotransferase activity coefficient; 4-PA, 4-pyridoxic acid; PLP, pyridoxal phosphate.
Correlations among vitamin B6 status indicators
Vitamin B6 intake was positively correlated with plasma PLP and urinary 4-PA and negatively correlated with EALT-AC and EAST-AC (Table 5). Plasma PLP was positively correlated with urinary 4-PA and negatively correlated with both EALT-AC and EAST-AC. Urinary 4-PA excretion was also negatively correlated with both EALT-AC and EAST-AC. EALT-AC was positively correlated with EAST-AC.
Table 5 Correlations among vitamin B6 status indicators of high school students aged 16–18 years * †
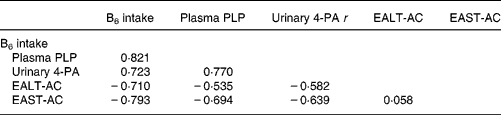
* For details of subjects and procedures, see Materials and methods.
† Based on the average of two values measured at the beginning and end of the semester for each student.
Pearson correlation coefficients (r) were all significant (P < 0·001).
EALT-AC, erythrocyte alanine aminotransferase activity coefficient; EAST-AC, erythrocyte aspartate aminotransferase activity coefficient; 4-PA, 4-pyridoxic acid; PLP, pyridoxal phosphate.
Vitamin B6 intake
The median intakes of vitamin B6 were determined from those who had adequate plasma PLP concentration, EALT-AC, EAST-AC and urinary 4-PA excretion (Table 6). The mean of median intakes of four vitamin B6 status indicators were 1·08 and 1·01 mg/d for boys and girls, respectively. The vitamin B6 requirements for high school boys and girls aged 16–18 years were suggested to be approximately 1·1 and 1·0 mg/d, respectively.
Table 6 Median intakes of vitamin B6 for boys and girls who had adequate vitamin B6 status indicators*

* For details of subjects and procedures, see Materials and methods.
† Based on the average of two values measured at the beginning and end of the semester for each student.
EALT-AC, erythrocyte alanine aminotransferase activity coefficient; EAST-AC, erythrocyte aspartate aminotransferase activity coefficient; 4-PA, 4-pyridoxic acid; PLP, pyridoxal phosphate.
Discussion
In the present study, median intakes of vitamin B6 at 1·08 and 1·01 mg/d for high school boys and girls aged 16–18 years, respectively, were determined on the basis of adequacy of PLP (>35 nmol/l), urinary 4-PA excretion (>3·0 mol/d), EALT-AC ( < 1·25) and EAST-AC ( < 1·8). The vitamin B6 requirements for these boys and girls should be approximately less than 1·1 and 1·0 mg/d, respectively, which are the levels set by the Dietary Reference Intake committee (USA). Monge-Rojas (Reference Monge-Rojas2001) reported that 50 percentiles of vitamin B6 intakes from prospective 3-d diet records were 1·3 and 1·2 mg/d for Costa Rican adolescents aged 12–19 years.
In the Navajo Health and Nutrition Survey, mean intakes of vitamin B6 were reported to be 1·5 (sd 0·1) mg/d obtained from a single 24-h diet recall for both sexes of adolescents aged 12–19 years, whereas the median intakes of vitamin B6 were 1·0 mg/d and below the RDA (Ballew et al. Reference Ballew, White, Strauss, Benson, Mendlein and Mokdad1997).
In the National Health and Nutrition Examination Survey, 1999–2000, mean intakes of vitamin B6 estimated from one 24-h dietary recall interview were reported to be 2·0 (sd 0·08) and 1·6 (sd 0·14) mg/d for US adolescent boys and girls aged 12–19 years, and the median intakes of vitamin B6 were 1·8 and 1·3 mg/d, respectively (Ervin et al. Reference Ervin, Wright, Wang and Kennedy-Stephenson2004). In their studies, only vitamin B6 intake data were provided and the vitamin B6 nutritional status was not determined. Therefore, the adequacy of vitamin B6 intake could not be determined without the vitamin B6 nutritional status from these studies.
Plasma PLP has been suggested to be the best single vitamin B6 status indicator (Liu et al. Reference Liu, Lumeng, Aronoff and Li1985; Institute of Medicine, 1998) because it appears to reflect tissue stores. Plasma vitamin B6 concentrations were found to be 199·6 (sd 57·9) and 186·0 (sd 47·1) nmol/l ( < 150 nmol/l is considered inadequate) in Nigerian adolescent boys and girls aged 16–18 years, respectively, with vitamin B6 intakes of 1·84 (sd 0·49) and 1·36 (sd 0·23) mg/d, indicating that these values of vitamin B6 intake may exceed the needs for these adolescents (Korede & Ajayi, Reference Korede and Ajayi1991).
In the present study, plasma PLP concentration of every student except one girl (28·7 nmol/l) was above 36 and 35 nmol/l for boys and girls, respectively, indicating adequate status and meets the cut-off values of 20 nmol/l for estimated average requirement (Institute of Medicine, 1998), 28·3 nmol/l by Driskell & Moak (Reference Driskell and Moak1986), 30 nmol/l by Leklem (Reference Leklem1990) and 34·4 nmol/l by Rose et al. (Reference Rose, Gyorgy, Butler, Andres, Norris, Shock, Tobin, Brin and Spiegel1976). All of the students in the present study (except one girl) had plasma PLP concentrations higher than any of the levels suggested as being indicative of vitamin B6 inadequacy by several researchers (Cleary et al. Reference Cleary, Lumeng and Li1975; Rose et al. Reference Rose, Gyorgy, Butler, Andres, Norris, Shock, Tobin, Brin and Spiegel1976; Shultz & Leklem, Reference Shultz and Leklem1987; Liu et al. Reference Liu, Lumeng, Aronoff and Li1985; Driskell & Moak, Reference Driskell and Moak1986; Hunt et al. Reference Hunt, Murphy, Martner-Hewes, Faraji, Swendseid, Reynolds, Sanchez and Mejia1987; Institute of Medicine, 1998). The mean intakes of vitamin B6 for total boys and girls were 1·04 (sd 0·29) and 0·96 (sd 0·28) mg/d, respectively, which were similar to the median intakes of 1·08 and 1·01 mg/d as determined by the present study. A total of 95 % boys and 94 % girls had urinary 4-PA excretion of >3·0 μmol/d with vitamin B6 intakes of 1·05 (sd 0·29) and 0·97 (sd 0·29) mg/d, respectively. These values were also comparable with those evaluated by the adequacy of plasma PLP reported in the present study.
Although urinary 4-PA excretion reflects current intake, it also provides complementary information in assessing vitamin B6 status. EALT-AC and EAST-AC are commonly used as measures of long-term vitamin B6 status. EALT-AC < 1·25 and EAST-AC < 1·8 are indicative of adequate status (Sauberlich et al. Reference Sauberlich, Dowdy and Skala1974; Leklem, Reference Leklem1990). In the present study, 23 % boy and 13 % girl students had inadequate B6 status, indicated by EAST-AC >1·25. Other researchers have reported that teenage females frequently had EAST-AC >1·16 as well as >1·25 (Kirksey et al. Reference Kirksey, Keaton, Abernathy and Greger1978, Suter et al. Reference Suter, Chrisley and Driskell1984, Driskell et al. Reference Driskell, Clark, Bazzarre, Chopin, McCoy, Kenney and Moak1985, Reference Driskell, Clark and Moak1987, Driskell & Moak, Reference Driskell and Moak1986). For students having adequate EALT-AC ( < 1·25) and EAST-AC ( < 1·8), mean dietary vitamin B6 intakes ranged from 0·98 (sd 0·28) to 1·16 (sd 0·26) mg/d, which were also comparable with those who had adequate plasma PLP.
Driskell et al. (Reference Driskell, Clark, Bazzarre, Chopin, McCoy, Kenney and Moak1985) estimated the mean daily vitamin B6 intake from food sources to be 1·20 (sd 0·06) mg for 583 adolescent girls aged 12, 14 and 16 years. Approximately 67 % of these adolescents had adequate vitamin B6 status as indicated by coenzyme stimulation of erythrocyte alanine aminotransferase activity. The mean coenzyme stimulation of erythrocyte alanine aminotransferase activity and PLP values of the adolescent girls aged 12, 14 and 16 years was 13·5 % and 45·2 nmol/l, with the estimated daily vitamin B6 intake of 1·25 (sd 0·04) mg (Driskell & Moak, Reference Driskell and Moak1986). Coenzyme stimulation values >25 % were observed in 18 % of these adolescents.
In the present study, mean plasma PLP levels of girls, being 57·2 (sd 19·3) nmol/l with mean B6 intake of 0·95 (sd 0·28) mg/d, were comparable with 78·1 (sd 19·3) nmol/l plasma PLP for 1·48 mg/d B6 intake in adolescent females reported by Chrisley et al. (Reference Chrisley, McNair and Driskell1991) using HPLC, whereas Driskell & Moak (Reference Driskell and Moak1986) used stimulation of tyrosine decarboxylase (l-tyrosine carboxylase) apoenzyme.
Recently, plasma PLP, urinary 4-PA, at least one indirect measure and the intakes of vitamin B6 and protein have been recommended for proper assessment of vitamin B6 status (Leklem, Reference Leklem1990). The present study determined the median intakes of vitamin B6 by using a combination of plasma PLP, urinary 4-PA, EALT-AC and EAST-AC and derived the suggestion for the vitamin B6 requirement. Vitamin B6 intake and status indicators were correlated in the present study. In addition, direct biomarkers of vitamin B6 intake (plasma PLP and urinary 4-PA excretion) were significantly related to functional indicators (EALT-AC and EAST-AC). Therefore, vitamin B6 intakes resulting in the adequacies of these direct and functional indicators were used to suggest the vitamin B6 requirement of high school students aged 16–18 years in the present study.
In conclusion, the median intakes of vitamin B6 for senior high school boys and girls were determined on the basis of adequate levels of plasma PLP, urinary 4-PA, EALT-AC and EAST-AC. We combined the four indicators and suggested that the vitamin B6 requirements were approximately 1·1 and 1·0 mg/d for high school boys and girls aged 16–18 years, respectively.
Acknowledgements
This study was supported in part by Department of Health (DOH89-TD-1055) and National Science Council (NSC89-2320-B-006-114), Taiwan.










