Background
Human milk is considered the gold standard of paediatric feeding during the first six months of life. However it is known there is a medical and social need to provide safe and effective alternative forms of nutrition for infants who cannot be fed with breast milk. As our knowledge and understanding of infant nutrition and physiology has increased, and formulae development technologies have became more sophisticated, recent efforts have focused on improving growth and development parameters in areas such as the immune, gastrointestinal or nervous systems. Nucleotides and nucleosides have an indispensable role in the structure of DNA and RNA. Various forms of ribonucleic acid found in cells and biological fluids are required intermediaries in energy metabolism, glycoconjugate synthesis and signal transduction, among other functionsReference Aggett, Leach, Rueda and MacLean1. Additionally, nucleotides have been identified as conditionally essential nutrients, since their de novo presence in rapidly growing tissues is both a functional and structural requirement for proper cell function. Animal studies show that the addition of nucleotides to experimental diets improves intestinal function and reduces the risk of Candida albicans and Staphylococcus aureus infections through modulation of the immune responseReference Ortega, Nuñez, Gil and Sanchez-Pozo2–Reference Uauy, Stringel, Thomas and Quan4. These animal studies supported the supplementation of milk-based infant formulae with ribonucleotides. Initially, supplementation was based on the “apparent” concentrations of nucleotides in breast milk (determined with analytical methods available at the time) and ranged from 12 to 33 mg/l (1·9 to 4·9 mg/418·4 kJ). Studies conducted with formulae supplemented at these levels showed statistically significant effects on weight gain, decrease in the risk of diarrhoea and improvement in certain immunological parametersReference Gil, Corral, Martinez and Molina5–Reference Cosgrove, Davies and Jenkins10. In 1995, Leach et al.Reference Leach, Baxter, Molitor, Ramstack and Masor11 pre-treated human milk samples by hydrolyzing the polymeric forms of ribonucleotides and ribonucleic acid adducts present in the sample to the corresponding ribonucleosides. The released total potentially available ribonucleoside content was subsequently quantified via HPLC. By conducting this analysis it became apparent that the biologically relevant concentrations of Total Potentially Available Nucleosides (TPAN) in human milk were higher than previously thought, at levels of 72 ± 24 mg/l (10·78 mg/418·4 kJ)Reference Leach, Baxter, Molitor, Ramstack and Masor11. Studies carried out with formulae supplemented with nucleosides at these TPAN levels showed similar results to those reported in previous publications regarding reduction of diarrhoea episodes but demonstrated higher antibody titres after vaccinationReference Pickering, Granoff and Erickson12–Reference Ostrom, Cordle and Schaller15, Reference Schaller, Kuchan, Thomas, Chordle, Winship, Buck, Baggs and Wheeler19–Reference Hawkes, Gibson, Roberton and Makrides21. No significant adverse events were observed in these studies. In November 2004 the Codex Committee on Nutrition and Foods for Special Delivery Uses (CCNFSDU), asked the Federation of International Societies of Paediatric Gastroenterology Hepatology and Nutrition (FISPGHAN) and the European Society (ESPHGAN) to join an International Expert Group (IEG) in the area of nutrition to discuss the optimum content of nutrients in infant formulae including ribonucleotide levelsReference Koletzko, Baker, Cleghorn, Neto, Gopalan, Hernell, Hock, Jirapinyo, Lonnerdal, Pencharz, Pzyrembel, Ramirez-Mayans, Shamir, Turck, Yamashiro and Zong-Yi16. The aim of this study is to systematically review the available evidence pertaining to formulae supplementation with ribonucleotides with the purpose of determining if the data support notions and positions on the biological activity and safety of these nutrients.
Objective
To review the available evidence to establish the efficacy, safety and dose-response effect of ribonucleotide-supplemented infant formulae.
Criteria for considering studies for this review
Types of studies
Only randomised controlled clinical trials (RCTs) comparing infant formulae containing ribonucleotides with formulae without nucleotides or breast milk were considered in this review.
Types of participants
Healthy children under 2 years of age, fed with formulae containing ribonucleotides vs. formulae without ribonucleotides or breast milk for at least one month. Studies with formulae that contained additional novel ingredients, other than nucleotides, were not included in this review.
Type of intervention
Groups of infants fed formulae containing nucleotides compared with groups fed with identical formulae without nucleotides, or groups fed with breast milk.
Outcome measures
The relevant outcome measures were humoral immune response (antibody titres) to common paediatric vaccinations, cellular immune response (total lymphocytes and lymphocyte subclasses and NK-cells), episodes of diarrhoea, and acute respiratory infections.
Search methods for identification of studies
A highly sensitive search strategy for the identification of controlled clinical trialsReference Moher, Schulz and Altman18 was used, in addition to using keywords (nucleotides OR nucleosides OR ribonucleotides OR ribonucleosides) AND [nutrition OR formulae]), limiting subjects to infants 0 to 24 months old. No limits for language or country of publication were set. The following databases were searched: PubMed (1966 to October 2005), Embase (1988 to October 2005), LILACS (1990 to October 2005), ARTEMISA (review of the 9th edition to December 2004), Cochrane Controlled Trials Register, Bandolier and DARE. Direct contact with the lead authors of detected publications was established to increase the chances of identifying sources in the so-called ‘grey literature’.
Methods of the review
Four evaluators working together extracted relevant data and carried out the methodological quality assessment for each of the studies. Disagreements were resolved by consensus. Standardised methods described by the Ibero American Cochrane Collaboration for preparing protocols were used, applying inclusion criteria, assessing the quality of publications and extracting information. Publication quality was determined using a point system described by Jadad using the CONSORT status as a guidelineReference Schaller, Kuchan, Thomas, Chordle, Winship, Buck, Baggs and Wheeler19. When information was deemed qualitatively and quantitatively relevant for the study, it was determined whether or not bio-statistical homogeneity criteria were met for inclusion in the study. Risk ratio for binary results and standarized mean differences for continuous findings were calculated. Results were combined using a model of random effects based on the weighted average of the results with proportional weights inverse to variance. For all estimates, a 99 % confidence interval (CI) was calculated. As necessary a heterogeneity test using Pearson's chi square test was performed with a p value of < 0·05 indicating significance and the possibility of publication bias was assessed using a funnel plot.
Results
Quantitative Synthesis of the Results
Fifteen studies were considered suitable for inclusion in the analysisReference Carver, Pimentel, Cox and Barness6–Reference Cosgrove, Davies and Jenkins10, Reference Pickering, Granoff and Erickson12–Reference Ostrom, Cordle and Schaller15, Reference Schaller, Kuchan, Thomas, Chordle, Winship, Buck, Baggs and Wheeler19–Reference Vazquez-Garibay, Mendez-Estrada and Romero-Velarde24. A total of six studies were excluded (three because ingredients other than nucleotides were not present at equal concentrations in experimental and control formulaeReference Martinez Agustin, Boza and Del Pino8, Reference Cosgrove, Davies and Jenkins10, Reference Lama More and Gonzalez13 and the remaining three because they were cohort or open label studiesReference Kuchan, Winship and Masor22–Reference Vazquez-Garibay, Mendez-Estrada and Romero-Velarde24). It was possible to identify important differences in immune function and clinical variables between groups. (Table 1)
Table 1 Quantitative results of the randomised controlled trials included in the meta-analysis

*URI = Upper respiratory infection reported (include otitis), ** Data non available, ¥ = Natural Killer cells, ≠ = Interleukin-2.
Meta-analysis results
Meta-analysis showed that nucleotide-fortified formulae compared to breast milk or control formulae showed a better antibody response to immunisation with Haemopillus influenza vaccine [SMD 1·74 (99 %CI 1·43, 2·05), P = 0·001], diphtheria toxin [SMD 0·94 (0·75, 1·12), P = 0·001] and oral polio vaccine [SMD 0·73 (0·51, 0·95), P = 0·001]; and fewer episodes of diarrhoea [RR 0·67 (0·58, 0·76), P = 0·02]. Regarding safety, we did not find differences in relation to the risk of upper respiratory infections [RR 1·11 (0·90, 1·36), P = 0·50]. (Figs. 1 & 4)

Fig. 1 Nucleotides and Hib-Abs production.
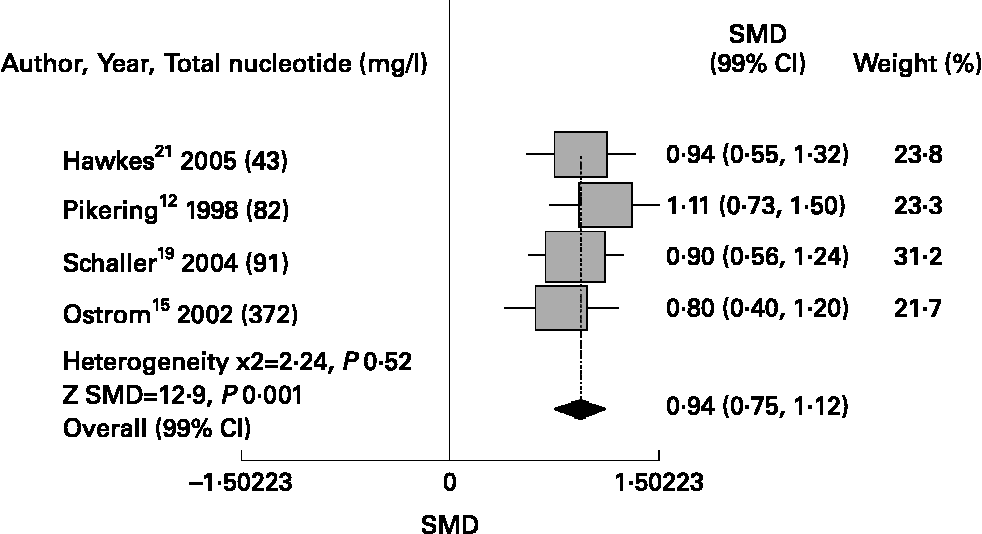
Fig. 2 Nucleotides and antibody response to diphtheria toxoid.

Fig. 3 Nucleotides and Antibody Response to Oral Polio Vaccine.

Fig. 4 Nucleotides and respiratory tract infections (Include otitis).
Recommendations
There is sufficient evidence to support the addition of nucleotides to infant formulae based on health benefit related findings, independently of the compositional evidence of their presence in human milk. Available evidence suggests that these benefits are conferred by the addition of 1·9 mg/418·4 kJ and are maintained or increased in studies with supplementation at 10·78 mg/418·4 kJ. Considerably greater effects for the selected endpoints and significantly larger clinical and bio-statistical differences among groups were observed at the higher levels of supplementation. The two main benefits supported by the studies considered in this analysis are improved maturation of the immune system and decrease in the incidence of diarrhoea. The evidence considered for the present analysis supports the supplementation of formulae at levels of at least 5 mg/418·4 kJ (33 mg/l) and with higher levels of 10·78 mg/418·4 kJ (72 mg/l), however, it is important to note that 50 % of the evidence from studies with formulae supplemented with 10·78 mg/418·4 kJ shows greater effects on endpoint variables. Other research groups have tested formulae with total nucleotide concentrations of 16 mg/418·4 kJ, which is recommended by the Panel of Experts of the Science Research Office. These concentrations have proven to be safe since there is no evidence of increase in the risk of infections or other related adverse events. Given the need to continue generating evidence and the potential beneficial impact on the health of infants, we recommend the continuing development of clinical trials of high methodological rigor testing nucleotide concentrations similar to those identified in breast milk through the TPAN analytical method. These studies would aim to establish the point at which the supplementation with higher concentrations of nucleotides does not represent a significant gain in health benefits. As in all trials involving infants and sole sources of nutrition, close surveillance of the occurrence of potential adverse events should be a high priority.
Conflict of interest statement
The authors have no conflict of interest to report. The work presented was supported by funds from the Pediatric Evidence Based Center COCHRANE-INP, Mexico. PG-C & GS-S participated by assembling the protocol, CJ & LD did the MEDLINE, EMBASE, LILACS and ARTEMISA search, CJ & LD built the data base and PG-C, JRM & GS-S did the statistical analysis & wrote the article.









