Spirulina, a blue-green filamentous alga which has been commercialised as a human food supplement, is gaining increasing attention for the treatment of various diseases because it contains abundant nutritional components(Reference Zhang, Xin and Li1). Experimental studies have suggested that Spirulina has benefits of antiviral, anticancer, and immune-strengthening effects(Reference Hayashi, Hayashi and Kojima2–Reference Hirahashi, Matsumoto and Hazeki4). However, little knowledge is available regarding the protective effect of Spirulina against liver injuries, and many existing findings are based on the study of individual nutrients that Spirulina contains, such as various antioxidants, rather than on direct research with Spirulina.
Oxidative and inflammatory pathways play important roles in d-galactosamine (d-GalN)- and acetaminophen (APAP)-induced hepatitis models(Reference Keppler, Lesch and Reutter5–Reference Blazka, Wilmer and Holladay8). It has been reported that thiobarbituric acid-reactive substances increase during the process of necrosis caused by d-GalN(Reference Sun, Hamagawa and Tsutui6) or APAP(Reference Jaeschke, Knight and Bajt7). IL-18 is a potent inflammatory cytokine which regulates autoimmune and inflammatory diseases(Reference Okamura, Tsutsui and Komatsu9). In our previous laboratory study(Reference Komano, Egashira and Sanada10), IL-18 expression was found to be elevated in d-GalN-induced hepatitis. However, it remains unclear whether IL-18 participates in APAP-induced liver injury.
In the present study, we have examined the effects of dietary Spirulina platensis on d-GalN- and APAP-induced liver injuries, respectively, as assessed by the activities of serum transaminases. Furthermore, hepatic thiobarbituric acid-reactive substances and IL-18 levels as well as serum IL-18 concentration were measured to investigate their potential involvement in the protective influence of S. platensis.
Experimental methods
Materials and chemicals
S. platensis was provided by ZaihuiShou Bio-engineering Co. (Eerduosi, China) and freeze-dried. APAP and sodium pentobarbital were purchased from Sigma Aldrich Japan (Tokyo, Japan). The SV Total RNA Isolation System was purchased from Promega Co. (Madison, WI, USA), and a First Strand cDNA Synthesis Kit for RT-PCR (AMV) was ordered from Roche Diagnostics (Mannheim, Germany). A SYBR® Premix Ex Taq™ II Kit (Perfect Real-time PCR) was obtained from Takara Bio Inc. (Otsu, Japan). A Mouse IL-18 ELISA Kit was purchased from Immuno-Biological Laboratories (Minneapolis, MN, USA). A transaminase C II-test Wako Kit and all other reagents were purchased from Wako Pure Chemicals Industries (Osaka, Japan).
Animals and diets
Male ICR mice, aged 5 weeks, were obtained from SLC Co. (Hamamatsu, Japan) and kept in an air-conditioned room (22 ± 1°C) with a 12 h light and dark cycle (light from 07.00 to 19.00 hours). The mice were acclimatised for 3 d with a commercial pellet diet (CE-2; CLEA, Tokyo, Japan) and for 4 d with a standard diet based on the American Institute of Nutrition (AIN)-93G formula(Reference Reeves, Nielsen and Fahey11) (g/kg: maize starch, 397·5; casein, 200·0; dextrinised maize starch, 132·0; sucrose, 100·0; soyabean oil, 70·0; cellulose, 50·0; vitamin mixture, 35·0; mineral mixture, 10·0; l-cystine, 3·0; choline bitartrate, 2·5; tert-butylhydroquinone, 0·014). The Spirulina diets, 3 % Spirulina, 6 % Spirulina and 9 % Spirulina, were prepared by adding 3, 6 or 9 % of Spirulina platensis at the expense of maize starch, casein and cellulose according to the protein and dietary fibre concentration in S. platensis (Reference Zhang, Xin and Li1), respectively. Butylated hydroxytoluene (BHT), which is a widely used phenolic antioxidant and proved to have hepatoprotective effects in a previous study of our laboratory(Reference Boindogurong, Higaki and Egashira12), was chosen as the diet supplement (0·5 %) at the expense of maize starch for the positive control group. All mice were allowed free access to diets and drinking water. The care and treatment of the mice were in accordance with The Ethical Guide for the Care and Use of Laboratory Animals, Chiba University, and the present study was approved by the Ethics Committee for Animal Experiments of Chiba University.
Hepatoprotective dose
In experiment 1, six groups of mice (six mice in each group) were fed with the standard diet (normal group A and negative control group B), the 0·5 % BHT diet (positive control group C), and the 3 % Spirulina (group D), 6 % Spirulina (group E) or 9 % Spirulina diet (group F), respectively. Acute hepatotoxicity was induced by intraperitoneal injection of d-GalN at a dose of 300 mg/kg body weight on day 7, while the mice in group A were injected with the same volume of 0·9 % saline. Diets were withheld for 4 h before and after d-GalN administration (8 h in total).
In experiment 2, six groups (group I to group VI) of mice (six in each group) were fed with the corresponding diets as in experiment 1, respectively. Acute hepatotoxicity in the mice was induced by intraperitoneal injection of APAP at a dose of 150 mg/kg body weight on day 7, while the mice in the normal group were injected with saline as in experiment 1. All mice were fasted for 16 h before and 6 h after APAP administration (22 h in total).
At 24 h after d-GalN or APAP treatment, the mice were anaesthetised by intraperitoneal injection of pentobarbital sodium at a dose of 50 mg/kg body weight and killed. Serum was obtained from blood to measure the glutamate oxaloacetoacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) activities, with a Transaminase C-II-test Wako Kit according to the manufacturer's instructions. The toxin doses, injection time and the fasting periods were chosen according to the results of our preliminary experiments (data not shown) and previous research (10,12) to establish effective liver injury models.
Effect on the hepatic thiobarbituric acid-reactive substances level
In experiment 3, three groups of mice (six mice in each group) were respectively fed with the standard diet (the normal and negative control groups) or the 6 % Spirulina diet (the treatment group) for 7 d, and the mice were intraperitoneally injected with saline or d-GalN and killed as in experiment 1. In experiment 4, the normal, negative control and treatment groups (six mice in each group) were fed with the standard diet or the 6 % Spirulina diet for 7 d, respectively, and the mice were intraperitoneally injected with saline or APAP and killed as in experiment 2. Serum was obtained and stored at − 20°C for transaminase (GOT, GPT) activity measurement and ELISA assay. Livers were quickly frozen in liquid N2 and kept at − 80°C. The hepatic thiobarbituric acid-reactive substances concentration was measured by the formation of malondialdehyde (MDA)(Reference Ohkawa, Ohishi and Yagi13).
Effect on IL-18 mRNA expression and serum IL-18 concentration
Total liver mRNA was extracted using the SV Total RNA Isolation System, and cDNA was synthesised from 1 μg RNA using a First Strand cDNA Synthesis Kit for RT-PCR (AMV). Real-time PCR was performed for IL-18 and the housekeeping gene, glyceladehyde-3-phosphate-dehydrogenase (GAPDH), using a SYBR® Premix Ex Taq™ II kit on the ABI PRISM 7000 Sequence Detection system (Applied Biosystems, Foster City, California, USA). The following primers were used: IL-18 (forward), 5′-GAA GAA AAT GGA GAC CTG G-3′; IL-18 (reverse), 5′-TTC ACA GAG AGG GTC ACA-3′; GAPDH (forward), 5′-CCA GGT TGT CTC CTG CGA CT-3′; GAPDH (reverse), 5′-ATA CCA GGA AAT GAG CTT GAC AAA GT-3′. Thermal cycling was initiated with a first incubation of 10 min at 95°C and followed by forty-five cycles of 30 s at 94°C, 1 min at 62°C, and 1 min at 72°C. Serum IL-18 concentration was measured by a mouse IL-18 ELISA kit from Immuno-Biological Laboratories. All the process was implemented according to the manufacturers' guidelines.
Statistical analysis
Results are expressed as mean values with their standard errors. The total variation was estimated by ANOVA followed by a Scheffe's multiple-comparison test with Excel Toukei (version 6.0; Esumi Co. Ltd, Tokyo, Japan). The level of statistical significance was P < 0·05.
Results
Hepatoprotective dose
No mortality in the experimental animals during the experimental period was observed. The final body weights (37·33 (se 0·76) to 38·83 (se 0·70) g), weight gains (5·17 (se 0·60) to 6·33 (se 0·67) g per 7 d) and food intakes (37·15 (se 0·66) to 39·37 (se 0·66) g per 7 d) were in the normal range for Slc:ICR mice of this age (7 weeks)(14), and showed no significant differences among the groups (Table 1).
Table 1 Effect of Spirulina platensis on body weights, food intakes and serum transaminase activities in mice treated with d-galactosamine (d-GalN) (experiment 1) or acetaminophen (APAP) (experiment 2)
(Mean values with their standard errors)

GOT, glutamate oxaloacetoacetate transaminase; GPT, glutamate pyruvate transaminase; A and I, standard diet; B and II, standard diet +d-GalN or APAP; C and III, 0·5 % butylated hydroxytoluene diet +d-GalN or APAP; D and IV, 3 % Spirulina diet +d-GalN or APAP; E and V, 6 % Spirulina diet +d-GalN or APAP; F and VI, 9 % Spirulina diet +d-GalN or APAP.
a,b,c,d Mean values within a column, within an experiment, with unlike superscript letters were significantly different (P < 0·05; n 6). The results were log-transformed before analysing by ANOVA.
Moreover, as shown in Table 1, the serum activities of GOT and GPT increased significantly in the mice administrated with d-GalN or APAP when compared with those in the corresponding normal groups, and supplementation with BHT or Spirulina diets suppressed the increase significantly. While the GOT and GPT activities were significantly reduced in all the Spirulina-treated groups compared with those in the corresponding negative control groups, treatment with the 6 or 9 % Spirulina diet showed more potent capability in decreasing the serum GOT and GPT levels compared with the 3 % Spirulina diet. With the consideration of no significant differences found in the 6 and 9 % Spirulina groups, the dose of 6 % Spirulina in the diet was selected for further studies.
Effect on the hepatic concentration of thiobarbituric acid-reactive substances
The MDA levels of the two negative control groups treated with d-GalN or APAP (Table 2) alone in experiment 3 and experiment 4 were significantly higher than those of the corresponding normal groups, which was the same as the changes of the serum GOT and GPT activities that kept in accordance with the previous experiments. Supplementation with dietary S. platensis significantly alleviated the increase of the MDA levels as well as the serum GOT and GPT activities in the two treatment groups (Table 2).
Table 2 Effect of Spirulina platensis on hepatic malondialdehyde (MDA), IL-18 mRNA expression and serum IL-18 concentration in mice treated with d-galactosamine (d-GalN) (experiment 3) or acetaminophen (APAP) (experiment 4)
(Mean values with their standard errors)
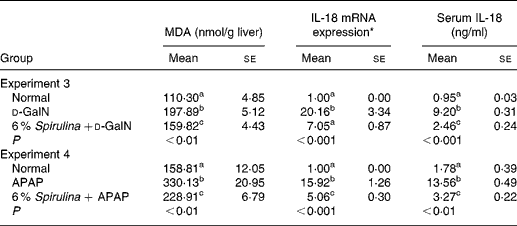
a,b,c Mean values within a column, within an experiment, with unlike superscript letters were significantly different (P < 0·05; n 6). The results were log-transformed before analysing by ANOVA.
* The values of IL-18 mRNA expression were presented relative to those of glyceladehyde-3-phosphate-dehydrogenase and normalised to 1 for the normal group.
Effect on the liver IL-18 mRNA expression and serum IL-18 concentration
Table 2 also shows that the liver IL-18 mRNA expression and serum IL-18 levels in the two negative control groups in experiment 3 and experiment 4 were significantly higher than those in the corresponding normal groups. The elevation of IL-18 expression in the liver and serum was significantly suppressed in the Spirulina treatment groups, roughly proportional to the reduction of liver MDA levels and serum activities of GOT and GPT.
Discussion
In the present study, the significant increase of serum GOT and GPT activities in the mice administrated with d-GalN or APAP was significantly suppressed by supplementation with Spirulina (6 or 9 %) or BHT in the diets, suggesting that dietary S. platensis could provide a significant protection against d-GalN- and APAP-induced liver injuries. The increase of MDA levels in the d-GalN- or APAP-intoxicated mice indicates d-GalN- or APAP-induced oxidative stress and lipid peroxidation. Treatment with dietary S. platensis significantly decreased the MDA levels and serum GOT and GPT activities, suggesting that S. platensis might scavenge reactive oxygen species generated from d-GalN or APAP intoxication and hence prevent hepatic cellular GOT and GPT from leaking into the blood(Reference Miyake15). Calculated by using the body surface area normalisation method(Reference Reagan-Shaw, Nihal and Ahmad16), which correlates well across several mammalian species with several parameters of biology including energy expenditure and basal metabolism, the effective dose of Spirulina (6 %) would be about 42 g for an average person (60 kg) per d. Since simplified production techniques have been set up to obtain large quantities of microalgae including S. platensis at competitive prices(Reference Peiretti and Meineri17), its daily application in the prevention of liver injury could be expected. However, further studies would be needed to investigate the effective composition of S. platensis and promote its uses in health care and medicine.
IL-18 is synthesised from a biologically inactive precursor and cleaved by the IL-1-converting enzyme caspase-1(Reference Ghayur, Banerjee and Hugunin18). As a result of activation of the caspase-1 pathway by oxidative stress or lipopolysaccharide stimulation, the production of mature IL-18 is increased(Reference Okamura, Tsutsui and Komatsu9). The present results showed that the increase of activities of GOT and GPT in d-GalN- or APAP-induced liver injury was accompanied by the elevation of IL-18 expression and lipid peroxidation. We supposed that the events might be triggered by oxidative stress, which caused necrosis and apoptosis and activated the caspase-1 pathway to promote IL-18 activation in Kupffer cells, and finally resulted in inflammatory damage and aggravated liver injuries. Moreover, IL-18 was found to be involved in APAP toxicity besides d-GalN-induced hepatitis in the present study, suggesting that IL-18 possibly is involved in a wide range of liver injuries.
In conclusion, dietary S. platensis could alleviate d-GalN- and APAP-induced liver injuries in mice significantly, and IL-18 and lipid peroxidation might be involved in the protective influence of S. platensis.
Acknowledgements
J. L. was supported by the National Oversea Study Scholarship ([2007]3021) from the China Scholarship Council and D.-F. R. was supported by a special programme (BLYX200935) of Beijing Forestry University.
J. L., D.-F. R., J.-Z. W., H. S. and Y. E. all designed the research; J. L., D.-F. R., H. S. and Y. E. analysed the data; J. L. and D.-F. R. performed the research and wrote the paper.
The authors declare no conflict of interest.






