Systemic lupus erythematosus is an autoimmune disorder affecting multiple organ systems, including the kidney, skin, lung, heart, haematopoietic system and brain(Reference Swaak, van den Brink and Smeenk1). Lupus nephritis (LN) is a frequent and serious complication of systemic lupus erythematosus that is directly related to the morbidity and mortality of patients(Reference Agrawal, Chiang and Rifkin2).
The production of autoantibodies against the nucleus and formation of glomerular immune deposits play a crucial role in the pathogenesis of LN and the local production of both cytokines and chemokines triggers glomerular inflammation, which ultimately leads to irreversible renal damage(Reference Mason and Berden3, Reference Mortensen, Fenton and Rekvig4). The interaction between B and T cells plays an especially dominant role in the progression of LN, leading to renal failure(Reference Bagavant and Fu5). B cells are often seen as the central cause of disease pathogenesis, which they induce by secreting autoantibodies(Reference Chan, Madaio and Shlomchik6). T cells are considered critical for the provision of help to B cells in the production of autoantibodies(Reference Wofsy and Seaman7). Therefore, the therapeutic target of LN has been suppression of the over-activated immune system via the use of high-dose glucocorticoids and immunosuppressants. However, only about 80 % of patients achieve renal remission in response to initial therapy using current treatment protocols; while approximately 30 % experience renal flares, 5–20 % of patients progress to end-stage kidney disease and treatment-related toxicity is appreciable(Reference Houssiau8). Thus, there is a need for alternative therapeutic options.
Recently, CD4+CD25+ regulatory T (Treg) cells have been shown to play a critical role in the maintenance of tolerance in the immune system(Reference Raimondi, Turner and Thomson9–Reference Nishimura, Sakihama and Setoguchi11). In addition, there is a great deal of convincing evidence that Treg cells are impaired in their suppressive function in patients with autoimmune diseases such as rheumatoid arthritis(Reference Minami, Sakai and Miyamura12), multiple sclerosis(Reference Viglietta, Baecher-Allan and Weiner13) and lupus(Reference Alvarado-Sanchez, Hernandez-Castro and Portales-Perez14).
Curcumin is a polyphenolic compound derived from the rhizomes of the plant Curcuma longa, which is used in Asian traditional medicine for its medicinal properties(Reference Ammon and Wahl15). The medicinal value of curcumin has been well recognised owing to its antioxidant, anti-tumour and anti-inflammatory activities. Additionally, recent studies have demonstrated that curcumin has the potential for use in the treatment of animal autoimmune disease models such as experimental allergic encephalomyelitis(Reference Natarajan and Bright16) and inflammatory bowel disease(Reference Sugimoto, Hanai and Tozawa17). These findings strongly suggest that curcumin may have a therapeutic effect on LN. In addition, recent clinical research demonstrated that oral supplementation of turmeric showed beneficial effects in LN patients(Reference Khajehdehi, Zanjaninejad and Aflaki18). However, the specific effect of curcumin on LN has not been well examined to date.
The present study was conducted to investigate the effects of curcumin on autoimmunity using New Zealand Black/White (NZB/W) F1 female mice. NZB/W mice spontaneously develop autoimmune disease as they age, which is characterised by lethal immune complex glomerulonephritis (GN), proteinuria and renal dysfunction. These symptoms in NZB/W mice closely resemble lupus in human subjects with severe GN(Reference Foster19). Therefore, we evaluated curcumin to determine if it could ameliorate renal inflammation, including immune complex-mediated GN with proteinuria and antinuclear autoantibody production. We found that renal disease improved remarkably in curcumin-treated mice when compared with control mice.
Materials and methods
Mice
NZB/W F1 female mice were purchased from Japan SLC, Inc. NZB/W F1 female mice spontaneously develop an autoimmune disease with immune complex GN, proteinuria and progression to renal insufficiency(Reference Foster19). All mice were housed under specific pathogen-free conditions during the experiments, which were conducted according to the ethical principles and guidelines established by Kyung Hee University for the care and use of experimental animals. At 18 weeks of age, mice were given curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), which was mixed in a normal diet at a concentration of 1 % (w/w). This dose of curcumin was selected based on previous studies in a murine skin carcinogenesis model(Reference Limtrakul, Lipigorngoson and Namwong20). All feeds were pelleted to avoid stratification and to ensure uniform feed and curcumin intake in the treated animals. All control and experimental diets were prepared every 2 weeks in our laboratory and stored at − 20°C until use.
Measurement of proteinuria
Beginning at 18 weeks of age, proteinuria was measured semi-quantitatively by impregnating wool paper test strips with spot urine (URiSCAN; Youngdong Pharmaceutical Company). The colour change of the strip infiltrated with the urine sample was visually judged against a standard strip and scored from 0 to 5+, according to the manufacturer's instructions. The urinary protein content was graded according to the score as follows: 0 = < 100 mg/l, 1+ = 100 mg/l, 2+ = 300 mg/l, 3+ = 1000 mg/l, 4+ = 3000 mg/l and 5+ = 10 000 mg/l.
Sera collection and autoantibody determination
At 18 and 32 weeks of age, mice were bled from the retro-orbital sinus following inhalation of isofluorane anaesthesia. A serum Ig class-specific antibody assay was conducted using mouse IgG1, IgG2a, IgG2b and IgG3 (Bethyl Laboratories, Inc.) ELISA kits. The serum anti-double-stranded DNA (dsDNA) IgG was evaluated using ELISA kits purchased from Alpha Diagnostic International at 32 weeks of age. All ELISA were conducted according to the manufacturer's instructions.
Immunofluorescent staining of IgG
Kidneys were embedded in Tissue-Tek OCT compound (Sakura), rapidly frozen using a Freezer Spray (Thermo Electron) and then cut into 20 μm-thick sections with a cryostat. The sections were subsequently permeablised for 20 min in PBS containing 0·5 % Triton X-100, after which the non-specific reactions were blocked and the samples were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (SantaCruz Biotechnology, 1:100) antibody at the optimal concentration. Finally, the fluorescence intensities within the glomeruli were measured using a confocal laser scanning microscope system (LSM 5 PASCAL, Carl Zeiss). Kidney sections were examined in each mouse, and at least ten kidney sections were analysed using the manufacturer's software (LSM 5 PASCAL release version 4.0 SP2; Carl Zeiss).
Histology and morphometric evaluation
At 32 weeks of age, the mice were killed for pathological evaluation. One-half of the left kidney from each mouse was fixed in 4 % paraformaldehyde solution, dehydrated in alcohol and embedded in paraffin. The kidney blocks were then cut into 5 μm-thick sections and stained with periodic acid–Schiff reagents. A total of twenty-five sequential glomeruli from the superior, middle and inferior cortices of each kidney were scored for damage by a pathologist in a single-blind fashion on a scale of 0–4+, where 0 = normal, 1+ = focal, mild or early proliferative, 2+ = multifocal proliferative with increased matrix and inflammatory cells, 3+ = diffuse proliferative and 4+ = extensive sclerosis/crescents.
Kidney TNF-α, IL-6 and monocyte chemoattractant protein-1
To examine pro-inflammatory molecules that were generated by nephritis, TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1) were measured in the mouse kidneys using ELISA (BD Biosciences). Briefly, the snap-frozen half of the left kidney tissue was homogenised in a PRO-PREP protein extraction solution (iNtRON Biotechnology, Inc.), incubated on ice for 20 min and then centrifuged at 13 000 rpm (4°C) for 15 min. The supernatant was used for a kidney pro-inflammatory cytokine assay. The protein concentrations in each supernatant were determined by a BCATH Protein Assay Kit (Thermo Scientific). The protein levels of the cytokines were corrected for the total amount of protein, after which the results were expressed as pg/mg or ng/mg.
Isolation of RNA and real-time PCR
Total cellular RNA was extracted using TRIzol reagent (Invitrogen Life, Technologies), according to the manufacturer's protocols, after which the concentration of total RNA was quantified by determining the optical density at 260 nm. Complementary DNA was synthesised using Transcriptor First Strand cDNA Synthesis Kit (Roche), according to the manufacturer's protocols, and then stored at − 20°C. Real-time quantitative PCR was conducted using LightCycler® 480 (Roche), employing SYBR Green I (Roche) as the dsDNA-specific binding dye for continuous fluorescence monitoring. Amplification was carried out using the reaction mixture, with a total volume of 20 μl, containing 375 nm of each gene-specific primer, 2 × PCR Master Mix (Applied Biosystems) and 2 μl of complementary DNA. The mixtures were then subjected to forty cycles of denaturation (95°C, 30 s), annealing and extension at 60°C, with fluorescence being measured at the end of each cycle. After the cycles were terminated, the signals at temperatures between 60 and 95°C were also collected to generate a dissociation curve. All reactions were conducted in duplicate to confirm reproducibility and a negative control was included (without template) to verify that no primer dimers were being generated. A standard curve for each primer was constructed using serial dilutions of complementary DNA, and the amount of target mRNA in each sample was normalised using that of the mean glyceraldehyde-3-phosphate dehydrogenase levels. The primer sequences are listed in Table 2.
Depletion of CD4+CD25+ regulatory T cells in vivo
Anti-mouse CD25 rat IgG1 (anti-CD25; clone PC61) were generated in-house from hybridomas obtained from American Type Culture Collection. A dose of 0·5 mg of anti-CD25 antibody was injected twice a week until the end of the experiment. The efficacy of CD4+CD25+ Treg cell depletion was confirmed by flow cytometry analysis, using phycoerythrin-anti-mouse CD25 and FITC-anti-mouse CD4 (eBioscience).
Statistical analysis
Statistical analysis of the data was conducted using Prism 4.02 software (GraphicPad Software, Inc.). Statistical analysis was conducted using Student's t test. Results with a P value < 0·05 were considered statistically significant.
Results
Outcome
Initially, ten mice were included in each group; however, three mice in the control group, two mice in the curcumin group, four mice in the anti-CD25 control group and three mice in the anti-CD25 curcumin group were dead before reaching 32 weeks of age. Data from these mice were excluded from the analysis of treatment efficacy. NZB/W mice treated with curcumin gained weight in a manner similar to that of mice provided with the normal diet (at 32 weeks of age, body weight: control 32·00 (sem 0·98) g v. curcumin 34·78 (sem 0·78) g). There were no signs of liver dysfunction in treated mice, as indicated by levels of serum aspartate aminotransferase and alanine aminotransferase being similar to the normal diet (at 32 weeks of age, aspartate aminotransferase: control 208·3 (sem 54·27) IU/l v. curcumin 127·1 (sem17·93)IU/l; alanine aminotransferase: control 57·00 (sem19·81) IU/l v. curcumin 36·43 (sem 4·54) IU/l).
Effect of curcumin on proteinuria
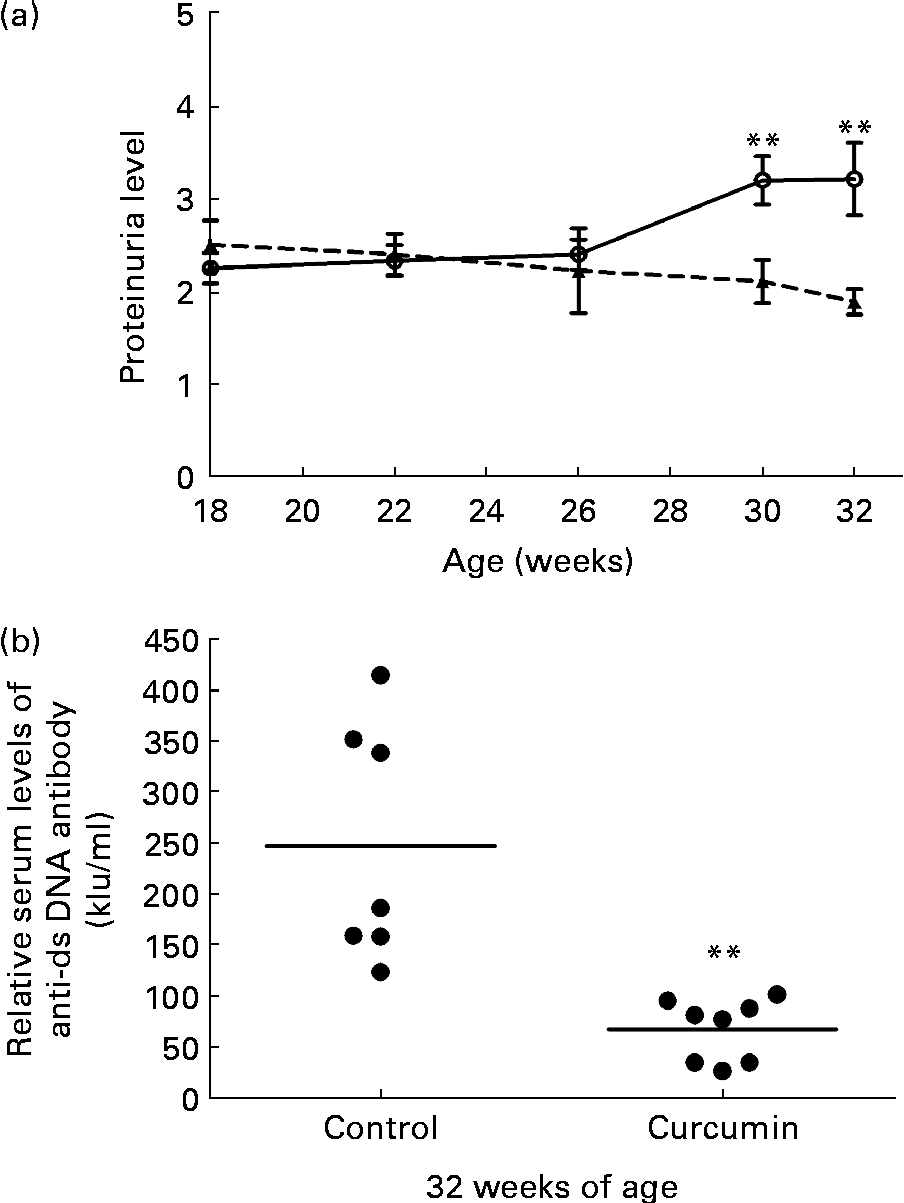
Significant proteinuria ( ≥ 2+ = 300 mg/l) was detected in 100 % of the mice in all groups prior to the start of the treatment. At 26 weeks of age, the mean level of urinary protein did not differ significantly between the two groups. At 30 weeks of age, the control group developed severe proteinuria. Conversely, the mean level of urinary protein in the curcumin-treated group began to decrease (control 3·2 (sem 0·26) v. curcumin 2·1 (sem 0·23); P< 0·01). By the end of the study, renal function had progressively deteriorated in the control group. In addition, urinary protein excretion was significantly attenuated in curcumin-treated mice (Fig. 1(a); control 3·2 (sem 0·39) v. curcumin 1·9 (sem 0·14); P< 0·01).

Fig. 1 Effect of curcumin (![]() ) on proteinuria and anti-double-stranded DNA (dsDNA). (a) Protein urea in New Zealand Black/White F1 mice at different weeks of age. (b) Anti-dsDNA IgG antibody was measured at 32 weeks of age. Values are means, with their standard errors represented by vertical bars (n 7–8). ** Mean value was significantly different from that of control mice (
) on proteinuria and anti-double-stranded DNA (dsDNA). (a) Protein urea in New Zealand Black/White F1 mice at different weeks of age. (b) Anti-dsDNA IgG antibody was measured at 32 weeks of age. Values are means, with their standard errors represented by vertical bars (n 7–8). ** Mean value was significantly different from that of control mice (![]() ) (P< 0·01; Student's t test).
) (P< 0·01; Student's t test).
Effect of curcumin on serum anti-double-stranded DNA IgG
The serum level of anti-dsDNA IgG was also determined at 32 weeks of age. Mice fed the normal diet had very high levels of anti-dsDNA antibodies in their serum. Anti-dsDNA antibody levels were diminished in the curcumin-treated mice (Fig. 1(b); control 250 (sem 44) kIU/ml v. curcumin 67 (sem 11) kIU/ml; P< 0·01).
Effect of curcumin on serum IgG isotypes
Serum levels of circulating IgG1, IgG2a, IgG2b and IgG3 were measured at 18 and 32 weeks of age. As expected, the levels of IgG1, IgG2a, IgG2b and IgG3 were significantly up-regulated in the control group at 32 weeks of age. However, the levels of IgG1 and IgG2a were significantly lower at the end of the treatment period in curcumin-treated mice when compared with the control mice. However, the levels of IgG2b and IgG3 were not significantly different between the groups (Table 1).
Table 1 Effect of curcumin on serum IgG isotypes† (Mean values with their standard errors, n 7–8)

Mean value was significantly different from that of control mice at 32 weeks of age: * P< 0·05, *** P< 0·001 (Student's t test).
† Serum IgG1, IgG2a, IgG2b and IgG3 were measured at 18 and 32 weeks of age.
Table 2 Primer sequences

Foxp3, forkhead box P3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effect of curcumin on immune complex deposits
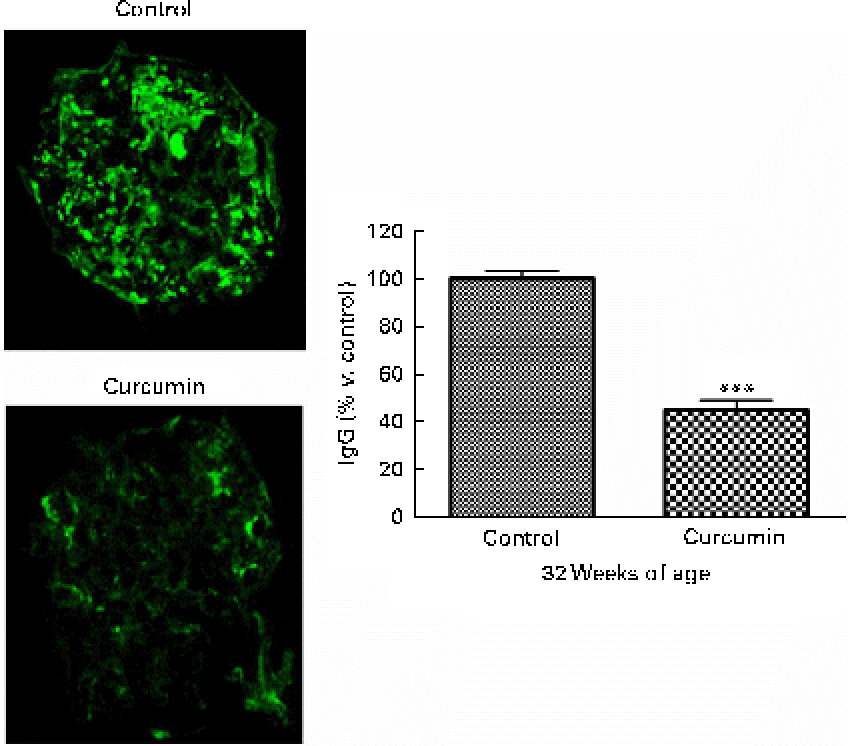
In LN, immune complex accumulation is believed to initiate inflammation. To understand how curcumin decreases GN, we assessed glomerular IgG binding by immunofluorescence microscopy. As shown in Fig. 3, a marked deposition of IgG was observed in the renal glomeruli of the control group. Blinded scoring of IgG staining intensity revealed increased IgG staining in the saline group when compared with the curcumin group, indicating that the amount of immune deposits present in the glomeruli was correlated with the damage observed by light microscopy (Fig. 2; control 100 (sem 3·2) v. curcumin 45 (sem 4·1); P< 0·001).

Fig. 2 Effect of curcumin on immune complex deposition in the glomeruli. Renal glomeruli were evaluated by staining kidney cryosections with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody. Fluorescence intensities within the glomerular capillary walls were detected using confocal microscopy. At least ten glomeruli per section were analysed (magnification: × 400). Values are means, with their standard errors represented by vertical bars (n 7–8). *** Mean value was significantly different from that of control mice (P< 0·001; Student's t test). (A colour version of this figure can be found online at www.journals.cambridge.org/bjn).
Effect of curcumin on renal histology
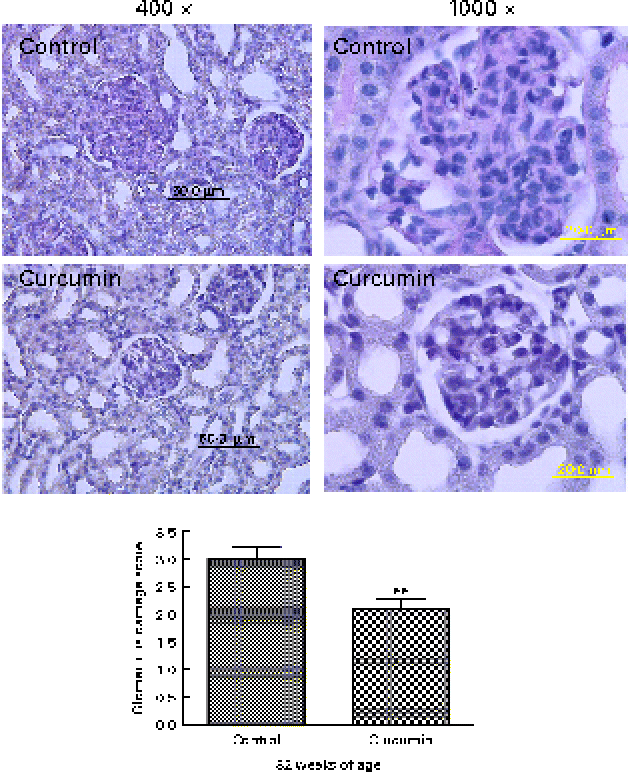
To evaluate the effects of curcumin on the development of GN, all surviving mice were killed at 32 weeks, after which kidney sections were examined by light microscopy. Glomeruli from the control group showed chronic GN, i.e. structural damage, including hyaline lesions, fibrinoid necrosis, cellular crescents, mesangial proliferation and mesangiolysis. In contrast, many glomeruli from the curcumin group showed mildly abnormal histology. To quantify these changes, we conducted blinded scoring of ‘glomerular damage’. The curcumin diet significantly reduced glomerular damage when compared with the normal diet (Fig. 3).

Fig. 3 Effect of curcumin on glomerulonephritis. Renal glomeruli from saline-treated mice and curcumin-treated mice (magnification: × 400 or × 1000). A total of twenty-five sequential glomeruli from the superior, middle and inferior cortices of each kidney were scored for damage by a pathologist in a single-blind fashion. Values are means, with their standard errors represented by vertical bars (n 7–8). ** Mean value was significantly different from that of control mice (P< 0·01; Student's t test). (A colour version of this figure can be found online at www.journals.cambridge.org/bjn)
Effect of curcumin on renal TNF-α, IL-6 and monocyte chemoattractant protein-1
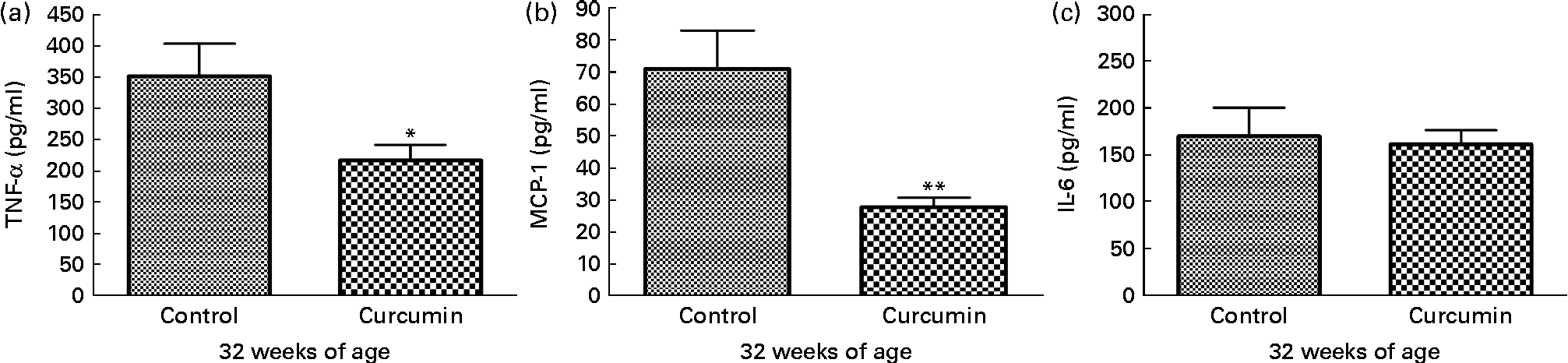
To examine pro-inflammatory molecules that were generated by nephritis, TNF-α, IL-6 and MCP-1 were measured in the kidneys of mice from each group. Control group mice showed increased levels of TNF-α and MCP-1 at 32 weeks of age. In contrast, the curcumin group showed reduced TNF-α (control 352·1 (sem 51·51) pg/mg and curcumin 203·4 (sem 15·10) pg/mg; P< 0·05) and MCP-1 (control 70·99 (sem 11·98) pg/mg and curcumin 28·51 (sem 3·176) pg/mg; P< 0·01) levels (Fig. 4). However, there was no significant difference in IL-6 level at 32 weeks of age.

Fig. 4 Effect of curcumin on (a) TNF-α, (b) monocyte chemoattractant protein-1 (MCP-1) and (c) IL-6 in kidney. The curcumin group showed a significantly reduced increase in TNF-α and MCP-1 when compared with the control group. Values are means, with their standard errors represented by vertical bars (n 7–8). Mean values were significantly different from that of control mice: * P< 0·05, ** P< 0·01 (Student's t test).
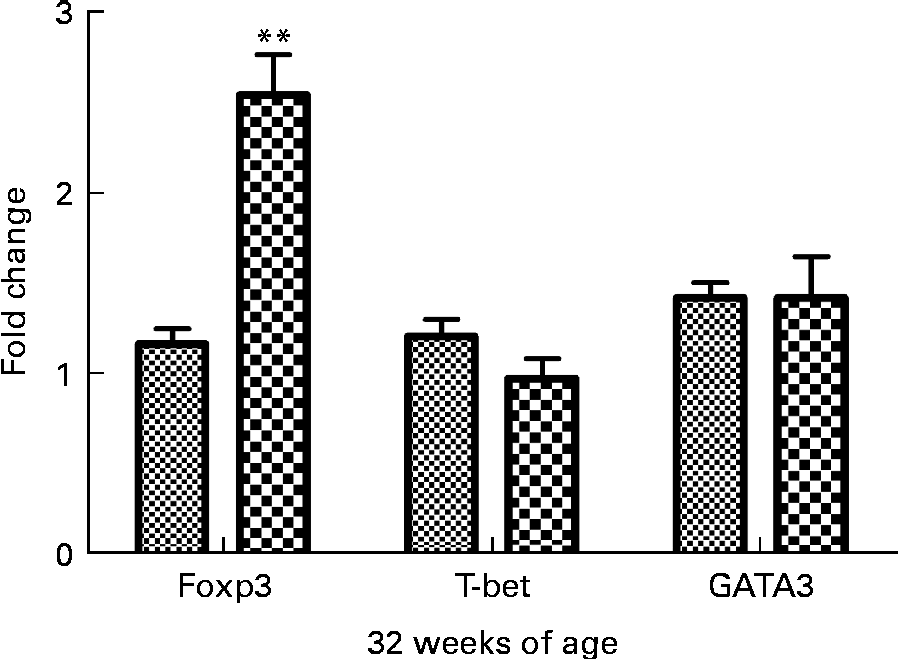
Effect of curcumin on T helper 1/T helper 2/Treg-specific transcription factor expression
To determine if curcumin treatment influenced helper T cell lineage development, we conducted real-time RT-PCR analysis of spleens isolated from 32-week-old mice fed a 1 % curcumin diet or normal diet for forkhead box P3 (Foxp3), T-bet and GATA-3. It is well-known that Foxp3 is the key transcription factor controlling Treg cell development and function(Reference Hori, Nomura and Sakaguchi21). T-bet is a transcription factor directing T helper 1 lineage commitment(Reference Szabo, Kim and Costa22). GATA-3 is a transcription factor maintaining T helper 2 cell function(Reference Yamashita, Ukai-Tadenuma and Miyamoto23). The results showed that Foxp3 expression was significantly increased in the curcumin group (P< 0·01, n 3) and the T-bet expression tended to be lower than in the control group (Fig. 5).

Fig. 5 Real-time PCR analysis of spleen in New Zealand Black/White mice. Real-time PCR was conducted in a Roche LightCycler® 480 (Roche) using SYBR Green I as the double-stranded DNA-specific binding dye and continuous fluorescence monitoring. Values are means, with their standard errors represented by vertical bars (n 3). ** Mean value was significantly different from that of control (![]() ) mice (P< 0·01; Student's t test).
) mice (P< 0·01; Student's t test). ![]() , Curcumin.
, Curcumin.
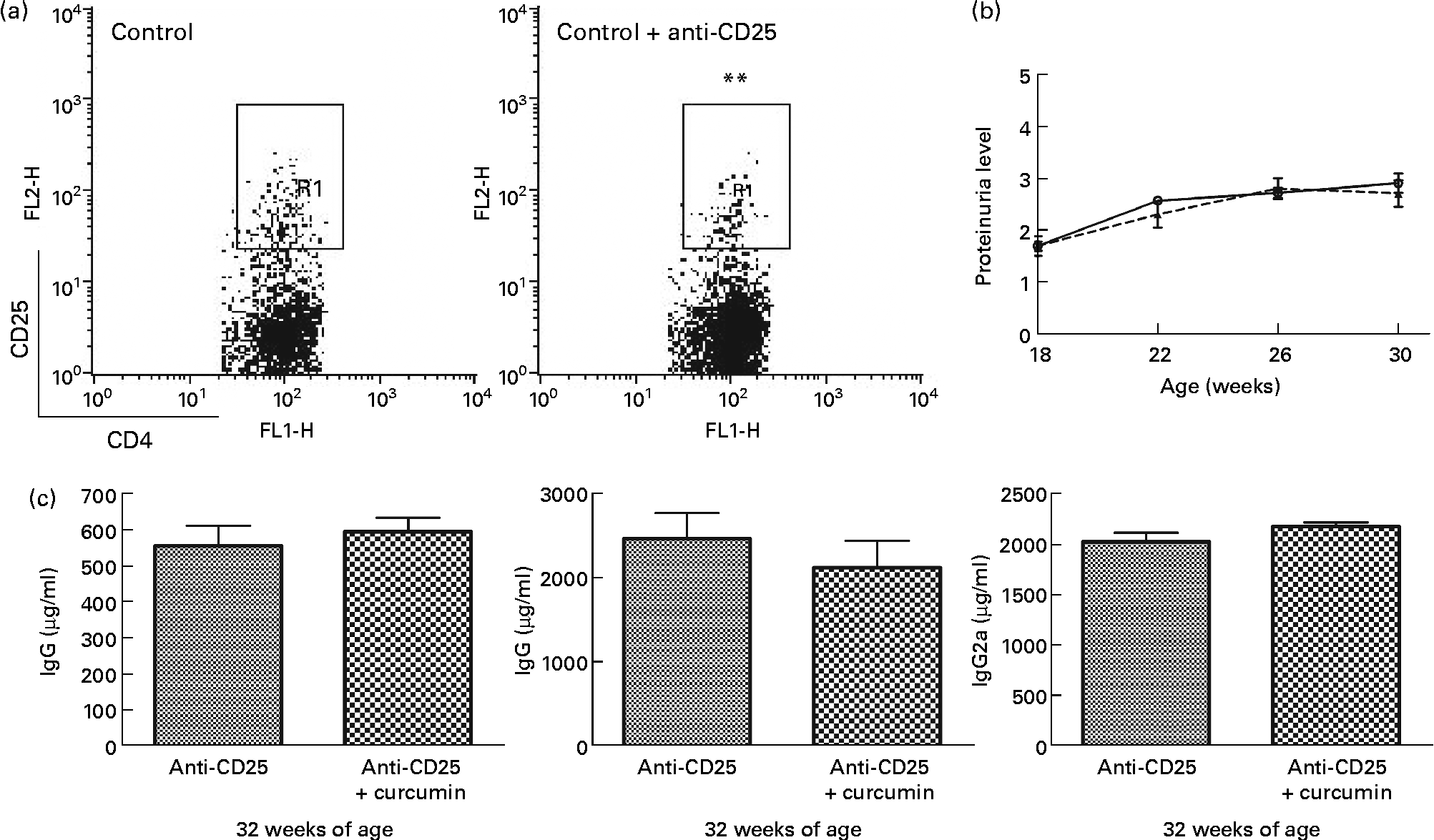
Effect of curcumin on regulatory T-depleted lupus-prone mice
Based on the Foxp3 mRNA expression, Treg cells appeared to have an extensive relationship with the therapeutic effect of curcumin in lupus-prone mice. We depleted Treg in NZB/W mice using PC61 and examined proteinuria and serum IgG isotype levels. Interestingly, the beneficial effects of curcumin disappeared (Fig. 6).

Fig. 6 Effect of anti-CD25 on New Zealand Black/White (NZB/W) mice. (a) A dose of 0·5 mg of anti-CD25 antibody was injected twice a week until the end of the experiment. The efficacy of CD4+CD25+ regulatory T cell depletion was confirmed by flow cytometry analysis. Gated on CD4+T cell (control n 3 (8·1 (sem 0·11) %), anti-CD25 n 15 (5·4 (sem 0·39) %)). ** Mean value was significantly different from that of control mice (P< 0·01). (b) Protein urea in NZB/W F1 mice at different weeks of age. (c) Serum IgG, IgG1and IgG2a were detected at 32 weeks of age (anti-CD25 (![]() ) n 7, anti-CD25+curcumin (
) n 7, anti-CD25+curcumin (![]() ) n 8). Values are means, with their standard errors represented by vertical bars. Data were analysed by Student's t test. FL1-H, fluorescence level 1-height; FL2-H, fluorescence level 2-height; R1, region 1.
) n 8). Values are means, with their standard errors represented by vertical bars. Data were analysed by Student's t test. FL1-H, fluorescence level 1-height; FL2-H, fluorescence level 2-height; R1, region 1.
Discussion
In the present study, we explored the therapeutic effects of curcumin on established LN in NZB/W F1 mice. Curcumin ameliorated the progression of renal diseases in NZB/W F1 mice. The proteinuria level and the serum levels of IgG1, IgG2a and anti-dsDNA IgG antibodies in the curcumin group were lower than those in the control group. Concomitant with serum IgG levels, IgG immune complex deposition in the glomeruli was reduced in curcumin-treated mice. Furthermore, renal inflammation was also decreased after curcumin treatment.
Hereditary lupus of NZB/W mice is an antibody-mediated systemic autoimmune disease in which T cells play a critical role in augmenting autoantibody secretion by B cells(Reference Wofsy and Seaman7). It is well known that when B cells come into contact with T helper 1 cells, they secrete IgG2a and IgG3(Reference Ando, Sercarz and Hahn24), but that upon interaction with T helper 2 cells, they produce IgG1 isotypes(Reference Stevens, Bossie and Sanders25). The present findings demonstrated that NZB/W mice treated with curcumin showed decreased levels of IgG1 and IgG2a. The production of IgG3 was slightly down-regulated. These results indicate that curcumin exerts immunosuppressive effects on both T helper 1 and T helper 2 immune responses and suggest that CD4+CD25+ Treg cells could be involved in this suppressive effect of curcumin. Indeed, the present study demonstrated that NZB/W mice treated with curcumin have up-regulated Foxp3 expression, which is a transcription factor specifically expressed in CD4+CD25+ Treg cells that programme its development and function(Reference Fontenot, Gavin and Rudensky26). Recently, CD4+CD25+ Treg cells were found to play a pivotal role in the maintenance of tolerance in rodents and human subjects(Reference Sakaguchi, Sakaguchi and Asano27). Additionally, many studies have provided compelling evidence that CD4+CD25+ Treg cells can re-establish self-tolerance and prevent autoimmune diseases(Reference Sakaguchi, Ono and Setoguchi10). It should be noted that Wolf et al. (Reference Wolf, Hochegger and Wolf28) provided strong evidence that CD4+CD25+ Treg cells are potent suppressors of anti-glomerular basement membrane GN in mice. It has also been reported that depletion of CD4+CD25+ Treg cells exacerbates the development of GN in NZB/W F1 mice(Reference Hayashi, Hasegawa and Adachi29). In the present study, the suppressive effect of curcumin on IgG1 and IgG2a production was inhibited after the elimination of CD4+CD25+ Treg cells. This result strongly indicates that delaying the disease pathogenesis in curcumin-treated mice is attributed to the direct or indirect effect of curcumin on CD4+CD25+ Treg cells. However, the present study is limited to the relation between Treg cells and curcumin, which confirmed that the Treg cell has a major therapeutic role in LN of NZB/W F1 mice. Curcumin treatment could affect other immune cells, which might cause a cascade effect to the Treg cells’ function. Though the present data strongly indicate that the immune-suppressive effect of curcumin is highly related to CD4+CD25+ Treg cells in LN, these results imply a further need to assess the mechanism of action of curcumin on individual immune cells and to elucidate the effect of curcumin on CD4+CD25+ Treg cells in LN.
Conclusion
Overall, the results of the present study document the therapeutic effect of dietary curcumin in the attenuation of renal manifestations of LN in NZB/W lupus mice for the first time. Although it is still necessary to elucidate the specific immune-modulating signalling mechanism of curcumin in LN, the findings presented herein indicate that protective effects of curcumin in LN may involve, at least in part, its interaction with CD4+CD25+ Treg cells.
Acknowledgements
The present work was supported by a Korea Science and Engineering Foundation grant funded by the Korea government (Ministry of Education, Science and Technology; MEST) (no. 2009-0063466). The authors declare no conflicts of interest. The work presented here was carried out in collaboration between all authors. H. L., H. K., G. L. and H.-S. C. defined the research theme. H. L. and H. K. designed methods and experiments, carried out the laboratory experiments, analysed the data, interpreted the results and wrote the paper. G. L. and H.-S. C. co-worked on associated data collection and their interpretation. H. B. co-designed experiments, discussed analyses, interpretation and presentation. All authors have contributed to, seen and approved the manuscript.