Type 1 taste receptors (T1Rs), α-gustducin and other taste signalling elements well known for their roles in taste transduction have recently gained additional attention for their function in extra-oral tissues. In the proximal small intestine, many taste signalling proteins have been found in enteroendocrine L cells, where they are involved in the secretion of glucagon-like peptide 1 (GLP-1) in response to sugars and non-energetic sweeteners( Reference Jang, Kokrashvili and Theodorakis 1 ). In the colon, where many GLP-1-producing cells also express α-gustducin, it is not known which gustducin-coupled receptors elicit the release of GLP-1. Interestingly, GLP-1 and other proglucagon gene products are also expressed in taste cells. Indeed, many gut hormones and neuropeptides (e.g. serotonin, cholecystokinin, vasoactive intestinal peptide and neuropeptide Y) are expressed in taste cells and act on neighbouring taste cells through paracrine effects( Reference Shen, Kaya and Zhao 2 – Reference Huang, Dando and Roper 4 ). GLP-1 released from taste cells modulates taste sensitivity to sweet compounds, and mice lacking the GLP-1 receptor exhibit reduced responses to sweeteners in behavioural assays( Reference Shin, Martin and Golden 5 ). Some taste receptor cells also express glucagon, and genetic or pharmacological disruption of glucagon signalling significantly reduces the taste preferences of mice for sugars( Reference Elson, Dotson and Egan 6 ).
The cephalic phase of digestive secretion, or ‘psychic reflex’, as Pavlov introduced it, is mediated by the actions of the central and peripheral nervous systems. It has been determined that oral exposure to food initiates the cephalic phase of digestion. The cephalic phase of feeding is a set of conditional reflexes regulated by taste, odour and visual stimuli. At the peripheral level, the cephalic phase is mediated by several hormones acting at the onset of digestive process before food is digested. Among the hormones shown to be released at this stage are insulin and ghrelin( Reference Power and Schulkin 7 , Reference Drazen, Vahl and D'Alessio 8 ). These hormones are thought to be released primarily from endocrine cells responding to nerve stimuli.
In the present study, we demonstrate that the secretion of GLP-1 from the taste cells themselves may contribute to the anticipatory cephalic phase. In particular, we demonstrate that sweet-sensing taste cells may provide important information about the carbohydrate content of food and help the organism prepare for post-cephalic phases of digestion through the secretion of GLP-1.
Materials and methods
Reagents
All the chemicals were purchased from Sigma-Aldrich or Invitrogen, unless otherwise specified.
Animals
Tas1r3 null (Tas1r3KO) mice have been described previously( Reference Damak, Rong and Yasumatsu 9 ). Mice were bred at Monell's animal facility. Wild-type (WT) controls were littermates or C57Bl/6 mice purchased from the Jackson Laboratory. All the mice, 12–16-week-old males, were maintained under a 12 h light–dark cycle and fed standard rodent chow. All the experimental protocols and procedures were approved by Monell's Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Immunohistochemistry of frozen circumvallate (CV) papilla sections was performed as described previously( Reference Yee, Sukumaran and Kotha 10 ). The anterior tip and lingual tissues containing the foliate and CV were dissected and placed in fresh 4 % paraformaldehyde for 1 h at 4C. Tissue samples were then transferred to an ascending series of 10–30 % sucrose (Sigma) for 2 d for cryoprotection. Tissues samples were briefly rinsed in 0·1 m-PBS solution (pH 7·4), mounted in Optimal Cutting Temperature medium from Tissue-Tek (OCT) and frozen in a 100 % alcohol ice bath. Frozen sections were cut at a thickness of 5 μm and mounted on Superfrost slides (Fisher). A double immunofluorescence technique was used to determine the co-localisation of GLP-1 and specific taste cell types. Labelling with primary antibodies was done sequentially, during which tissue samples were incubated with anti-GLP-1 and labelled first, followed by a second labelling procedure with primary antibodies for specific taste cell types. Briefly, 5 μm frozen sections were dried in an oven at 45°C for 15 min and then rehydrated with 0·1 m-PBS solution (pH 7·4)+0·1 % Triton X-100 (PBST). A mouse-on-mouse kit was used (BMK-2202; Vector Laboratories) to label mouse anti-GLP-1 (1:100, G2040-13Q; US Biological). The mouse anti-GLP-1 antibody is directed against a full-length peptide (31 amino acid (aa)) corresponding to human GLP-1 according to the manufacturer's datasheet. Following the manufacturer's protocol, a secondary streptavidin Dylight 488 antibody (1:500, SA-5488; Vector) was used for detection. Tissue samples were then washed with PBST three times for 1 h with shaking, and non-specific binding was blocked with Superblock neat (Pierce) at room temperature for 1–2 h. Specific taste cell type markers were used to label type I cells (rabbit anti-triphosphate diphosphohydrolase-2 (NTPDase2), 1:500; Centre de Recherche du CHUL), type II cells (guinea pig anti-TrpM5, 1:500; gift from Dr Emily Liman; goat anti-T1R3, 1:250, sc-25 458; Santa Cruz Biotechnology) and type III cells (rabbit anti-5HT, 1:500, no. 20 080; Immunostar). For serotonin detection, the mice were injected with 5-hydroxy-l-tryptophan (H9772; Sigma) 1 h before being killed. Tissue samples were incubated with primary antibodies for specific taste cell types overnight at 4°C in a humidified chamber. After three 20 min washes with PBST, tissue samples were incubated for 1 h at room temperature with one of the following fluorescent secondary antibodies (1:300–500) in PBS: Dylight 647 donkey anti-rabbit (Jackson ImmunoResearch) for immunofluorescence of rabbit primaries; Alexa 647 donkey anti-goat (Invitrogen) for immunofluorescence of goat primaries; Dylight 647 donkey anti-guinea pig (Jackson ImmunoResearch) for immunofluorescence of guinea pig primaries. Tissue samples were washed with PBST three times for 1 h with shaking and then rinsed with distilled water for 10 min. Nuclei were labelled with 4′,6-diamidino-2-phenylindole (DAPI, 1:1000; Invitrogen) for 5 min for cell counting purposes. The slides were mounted with Vectashield (Vector). Supplemental Fig. S1 (available online) shows the positive and negative controls for the primary and secondary antibodies for GLP-1. A positive control assay was carried out on mouse pancreas, and expected labelling of the α-cells in the islets of Langerhans was observed. Negative control assays were carried out with anti-GLP and the omission of the secondary antibody and the omission of primary antibody and streptavidin Dylight 488 anti-mouse secondary antibody and anti-GLP-1 with various secondary antibodies to show non-cross reactivity. No anti-GLP-1 labelling was observed under any of the negative control conditions.
Imaging
Double immunofluorescence images were captured with Leica TCS SP2 Spectral Confocal Microscope (Leica Microsystems, Inc.) using UV, Ar, GeNe and HeNe lasers and appropriate excitation spectra. The Leica Scanware software was used to acquire Z-series stacks captured at a 0·25–0·35 μm step size. Images were scanned in a 512X512 pixel format with four lines plus one frame averaging. The brightness and contrast of the digital images were adjusted linearly for background standardisation and arranged using Photoshop CS (Adobe Systems, Inc.). For negative controls, all the images were taken with the same acquisition settings (i.e. laser power, gain, offset and photomultiplier tube (PMT) settings) used for positive labelling for each secondary antibody. No adjustments of brightness or contrast were made to the positive and negative control images.
Cell counting
For cell counting, three to four sections of foliate and CV papillae from two WT mice and six to ten sections of fungiform papillae from one WT mouse were used. Sections spaced over 60 μm were selected and only taste cells with identifiable DAPI-labelled nuclei were considered. A small Z-stack of a thickness between 3 and 4 μm was imaged at a magnification of 20 × with digital zoom in which individual taste cells can be clearly identified. Cells were counted manually in Photoshop, which allows the visualisation of each red, green, blue (RGB) channel separately to confirm co-localisation.
Tastant application
The mice were anaesthetised using an intraperitoneal injection of Avertin (125 mg per kg of body weight). Unconscious mice were placed on a warm (37°C) plate to maintain body temperature, the mouth was opened and the tongue was pulled out slightly with blunt-end forceps to get access to the posterior portion. A small piece of Whatman filter paper (10 mm × 4 mm) soaked in water (Control) or water containing 100 mm-glucose was placed on top of the posterior tongue. This concentration of glucose has been shown to be effective at stimulating behavioural and nerve responses in mice( Reference Damak, Rong and Yasumatsu 9 ).
Surgery
For unilateral cervical vagotomy, anaesthetised mice were laid on their back and an approximately 1·5 cm-long incision was made on the right side of the neck. Muscles were retracted to get access to the carotid trunk. The vagus nerve was gently separated from the carotid artery and cut in cross-section. For oesophagectomy/truncal vagotomy, anaesthetised mice were laparotomised, organs were retracted, and oesophagus was revealed. The oesophagus was tightly ligated with a silk suture approximately 1 cm above the gastro-oesophageal junction and cut circumferentially between the suture and the gastro-oesophageal junction such that the proximal portion was separated entirely from the gastric portion.
Blood collection
Tastants were applied on the posterior tongue as described, and blood was collected with a heparin-coated fine glass pipette from the orbital sinus of surgically intact mice and mice in which unilateral cervical vagotomy was performed. In mice that had undergone oesophagectomy/truncal vagotomy, blood was collected from the hepatic portal vein. Blood samples were centrifuged, dipeptidyl peptidase-4 (DPP4) inhibitor was added and stored on − 80°C for GLP-1 measurements.
Taste papilla explants
Explants of CV papilla taste tissue were prepared as described previously( Reference Ozdener, Yee and Cao 11 ). CV papilla explants were incubated for 2 h in Iscove's modified Dulbecco's medium (IMDM) buffer (control samples) or in IMDM buffer containing 100 mm-glucose. The medium was collected and stored at − 80°C for GLP-1 measurements. Each CV papilla consists of two bean-shaped parts of equal sizes. To ensure a similar number of taste buds and taste cells in all the preparations, CV papillae were bisected with a razor blade, one half was used as a control and the other half was incubated with a glucose/tastant-containing medium.
Glucagon-like peptide 1 immunoassay
GLP-1 levels in the blood or medium were measured using a GLP-1 ELISA sensitive for the active form of mouse/rat GLP-1 (Linco). Determinations were done in duplicate for the numbers of mice indicated.
Statistical analyses
Significance was examined using either the unpaired t test or multiple-comparisons ANOVA and post-test, as appropriate. All the data are presented as means with their standard errors; P< 0·05 was considered to be statistically significant. Graphics as well as statistical analyses were performed using GraphPad Prism (GraphPad Software).
Results
Taste cells express and secrete glucagon-like peptide 1
Given the presence of taste signalling proteins in enteroendocrine cells( Reference Jang, Kokrashvili and Theodorakis 1 ) and the presence of multiple gut hormones in taste cells( Reference Shin, Martin and Kim 12 , Reference Acosta, Hurtado and Gorbatyuk 13 ), we sought to determine whether taste cells directly release hormones into the bloodstream as part of the cephalic phase of hormone release. We focused on the secretion of GLP-1 by taste cells, which has previously been shown to be expressed in certain type II taste cells( Reference Shin, Martin and Golden 5 ).
To determine which types of taste cells express GLP-1, we performed double immunohistochemistry with markers specific for the taste cells of types I, II and III. Double immunohistochemistry of fungiform, foliate and CV papilla sections revealed that GLP-1 was most often co-expressed in type II taste cells as assessed by co-expression with TrpM5 (Fig. 1(d)–(f))). In posterior taste fields, approximately 91–93 % of the GLP-1-expressing cells also expressed TrpM5, although they constituted only about 20–27 % of the total type II taste cells (Table 1). In fungiform papillae, only approximately 23 % of the GLP-1-expressing cells also expressed TrpM5. Double immunostaining for the GLP-1- and T1R3-expressing subset of type II taste cells (Fig. 1((g)–(i)) revealed that only approximately 13–35 % of the GLP-1-expressing cells also expressed T1R3 (Table 2), indicating that among type II taste cells, it is more often the cells that do not express T1R3 that express GLP-1. Double immunostaining for NTPDase2 and GLP-1 revealed that GLP-1 is generally not expressed in type I taste cells (Fig. 1((a)–(c))). Double immunostaining for serotonin 5-hydroxyltryptamine (5HT) and GLP-1 indicated that approximately 6–17 % of the GLP-1-expressing cells expressed serotonin (Fig. 1((j)–(l); Table 3). The present results are in contrast to those reported by Shin et al. ( Reference Shin, Martin and Golden 5 ), who found that in CV taste cells approximately 52 % of the GLP-1-expressing cells expressed T1R3 and approximately 44 % expressed serotonin.

Fig. 1 Expression of glucagon-like peptide 1 (GLP-1) in taste cell types in anterior and posterior taste fields. Mouse fungiform, foliate and circumvallate papilla sections were double-stained with antibodies against GLP-1 and markers for taste cell subtypes. (a–c) Double staining for GLP-1 (green) and the type I cell marker NTPDase2 (red) revealed no doubly positive taste cells. (d–f) Double staining for GLP-1 (green) and the type II cell marker TrpM5 (red) revealed frequent doubly positive taste cells. (g–i) Double staining for GLP-1 (green) and the type II cell sweet/umami receptor subunit T1R3 (red) revealed a few doubly positive taste cells. (j–l) Double staining for GLP-1 (green) and the type III cell marker serotonin (5HT) (red) revealed occasional doubly positive taste cells. Arrows indicate double-stained GLP-1+/5HT+ type III taste cells. 4′,6-Diamidino-2-phenylindole (DAPI) (blue) was used to label cell nuclei. Scale bars = 20 μm.
Table 1 Numbers of mouse taste cells expressing glucagon-like peptide 1 (GLP-1) and TrpM5*
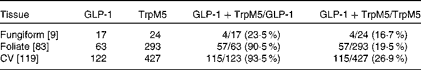
CV, circumvallate.
* Taste cells in fungiform, foliate and CV papillae were double-stained for GLP-1 and TrpM5. Then, doubly labelled taste cells were counted in the fungiform, foliate and CV papillae. The number of taste buds counted is given in brackets. The percentage of co-expression is given in parentheses.
Table 2 Numbers of mouse taste cells expressing glucagon-like peptide 1 (GLP-1) and type 1 taste receptor 3 (T1R3)*
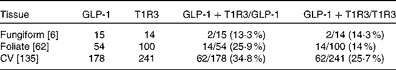
CV, circumvallate.
* Taste cells in fungiform, foliate and CV papillae were double-stained for GLP-1 and T1R3. Then, doubly labelled taste cells in the fungiform, foliate and CV papillae were counted. The number of taste buds counted is given in brackets. The percentage of co-expression is given in parentheses.
Table 3 Numbers of taste cells expressing glucagon-like peptide 1 (GLP-1) and serotonin (5HT)*
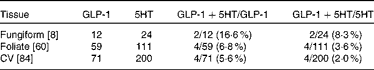
CV, circumvallate.
* Taste cells in fungiform, foliate and CV papillae were double-stained for GLP-1 and 5HT. Then, doubly labelled taste cells in the fungiform, foliate and CV papillae were counted. The number of taste buds counted is given in brackets. The percentage of co-expression is given in parentheses.
We measured glucose-elicited release of GLP-1 from CV papilla explants from WT mice or knockout mice lacking Tas1r3 (Tas1r3KO) in the culture medium. Glucose elicited the release of GLP-1 from CV papilla explants from both WT and Tas1r3KO mice (Fig. 2(a)). To determine whether taste cells act as endocrine cells to release hormones into the circulation, we measured the effects of oral stimulation with glucose on GLP-1 secretion in WT and Tas1r3KO mice. We measured blood GLP-1 levels before stimulation and 10 and 20 min after oral stimulation with glucose. In WT mice, oral stimulation with glucose induced significant release of GLP-1 after glucose application on the tongue (20 and 40 % above baseline at 10 and 20 min, respectively), compared with the control, non-stimulated mice (Fig. 2(b)). In addition, at 20 min, glucose induced the release of GLP-1 in Tas1r3KO mice (Fig. 2(b)), indicating that the T1R2+T1R3 sweet taste receptor is not required for glucose-stimulated GLP-1 release from taste cells.

Fig. 2 Release of glucagon-like peptide 1 (GLP-1) from taste cells. (a) Glucose-elicited release of GLP-1 in vitro from circumvallate papilla explants of wild-type (WT) and Tas1r3 knockout (Tas1r3KO) mice. Explants were incubated for 2 h in Iscove's modified Dulbecco's medium (IMDM) buffer (control, □) or IMDM buffer containing 100 mm-glucose (■). Values are means for four explants for each experiment, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control mice (P< 0·05). (b) Secretion of GLP-1 in intact mice after oral stimulation with glucose. WT and Tas1r3KO mice were stimulated by oral application of water or 100 mm-glucose. Blood was collected 10 min (■) and 20 min (□) after oral stimulation. ΔF/F indicates the percentage change in blood GLP-1 levels v. basal level (0 min, not shown). Values are means for four mice for each experiment, with standard errors represented by vertical bars. † Mean value was significantly different from that observed after 10 min stimulation (P< 0·05). Mean value was significantly different from that obtained for the WT mice stimulated with water at the same time point: ‡ P< 0·05, ‡‡ P< 0·01.
Unilateral cervical vagotomy reduces vagally mediated effects but does not eliminate cephalic-phase hormone release through the activation of vagal efferent fibres in response to food-related sensory stimuli. In intact WT mice and ones that had been cervically vagotomised unilaterally, oral stimulation with glucose elicited an increase in circulating GLP-1 levels (Fig. 3(a) and (b)). However, in the unilaterally vagotomised mice, this effect could be mediated by the remaining intact vagus nerve acting to stimulate the release of GLP-1 from gastrointestinal endocrine cells. To completely exclude vagal effects on the release of GLP-1 after oral stimulation, we monitored circulating GLP-1 levels in mice after a combined procedure of truncal vagotomy and oesophagectomy. This procedure dissects both branches of the vagus nerve below the oesophageal hiatus and thus eliminates efferent effects from the gastric and pancreatic branches of the vagus, as well as from vagal branches innervating the small and large intestines. We found that oral stimulation with glucose in mice in which truncal resection of the vagus was performed still elicited the release of GLP-1 (Fig. 3(c)). Both unilateral cervical vagotomy and truncal vagotomy reduced baseline blood GLP-1 levels 20 min after nerve resection with a more pronounced effect from the truncal vagotomy (Fig. 3(d)). However, oral stimulation with glucose in mice in which truncal vagotomy was performed still elicited a significant increase in blood GLP-1 levels at 10 and 20 min (Fig. 3(c)). Comparison of the GLP-1 responses in intact v. vagotomised mice by two-tailed t test indicated that differences were significant at both 10 min (P= 0·034) and 20 min (P= 0·032). This increase in GLP-1 levels is independent of vagal efferent actions and thus may be the result of direct release of GLP-1 from endocrine taste cells into the bloodstream. Consistent with this inference is our observation that CV tissue explants can release GLP-1 into the culture medium upon stimulation with glucose (Fig. 2(a)).

Fig. 3 Secretion of glucagon-like peptide 1 (GLP-1) from taste cells in vagotomised mice. (a) Intact wild-type mice were stimulated by oral application of water (□) or 100 mm-glucose (■) and then blood was collected before (0 min) and 10 or 20 min after oral stimulation and assayed for blood GLP-1 levels. (b, c) Same procedures as in (a) were used, except that unilateral cervical vagotomy (b) or oesophagectomy/truncal vagotomy (c) was performed in mice before oral stimulation. (d) Baseline blood GLP-1 levels 20 min after surgical procedures (intact mice, unilaterally vagotomised mice (unilateral V) and truncally vagotomised mice (truncal V)). Values are means for five mice for each experiment, with standard errors represented by vertical bars. * Mean value was significantly different from that obtained after stimulation with water (P< 0·05). Mean value was significantly different from that observed at time 0 min: † P< 0·05; †† P< 0·01; ††† P< 0·005. ‡‡ Mean value was significantly different from that of intact mice (P< 0·01). § Mean value was significantly different from that of unilaterally vagotomised mice (P< 0·05).
Discussion
A number of ‘gut’ hormones have been found to be expressed in taste cells( Reference Shen, Kaya and Zhao 2 – Reference Shin, Martin and Golden 5 ). Notably, the incretin GLP-1 that is co-expressed with taste receptors and gustducin in enteroendocrine cells in the gut( Reference Jang, Kokrashvili and Theodorakis 1 ) is also expressed in taste cells( Reference Shin, Martin and Golden 5 ). In the present study, we observed most of the GLP-1-expressing taste cells in posterior taste fields to be TrpM5-expressing type II receptor cells. Only a small number of GLP-1-expressing cells were found among type III cells marked by the expression of serotonin. Among type II taste cells, GLP-1 was most often found in the TrpM5-positive/T1R3-negative cells: these are likely to be α-gustducin-positive cells. Among the GLP-1-expressing taste cells in CV papillae, Shin et al. ( Reference Shin, Martin and Golden 5 ) found that between 44 and 56 % also expressed serotonin, T1R3 or α-gustducin. At present, we cannot explain the differences in co-expressing cells in the present study v. those in the study of Shin et al.; we did not examine the co-expression of α-gustducin with GLP-1.
Mice that lack the sweet receptor subunit T1R3 have little or no chorda tympani nerve response to non-energetic sweeteners, but still respond normally to sugars( Reference Damak, Rong and Yasumatsu 9 ). That taste cells also express sugar sensors and transporters typically found in the intestine and pancreas (e.g. sodium glucose cotransporter-1 (SGLT1), GLUT and KATP) may explain how Tas1r3 knockout mice can still respond to sugars( Reference Yee, Sukumaran and Kotha 10 ). In the present study, we found that glucose stimulates the release of GLP-1 from taste cells. Moreover, mice lacking Tas1r3 were found to secrete GLP-1 in response to orally applied glucose, indicating that the release of GLP-1 from taste cells may be mediated independently of T1R2+T1R3 sweet receptors, e.g. by sugar transporters and KATP shown to be present in T1R3-positive taste cells( Reference Yee, Sukumaran and Kotha 10 ).
Food digestion is often divided into multiple phases, e.g. neural/cephalic, gastric and intestinal. The neural/cephalic phase of digestion is a set of physiological, endocrine and autonomic responses of the digestive system that result from the stimulation of sensory systems at the cephalic level( Reference Zafra, Molina and Puerto 14 ). Cephalic-phase responses are well established for insulin and ghrelin( Reference Power and Schulkin 7 , Reference Drazen, Vahl and D'Alessio 8 ), but controversial or thought not to occur with the incretin hormones GLP-1 and glucose-dependent insulinotropic peptide( Reference Ahrén and Holst 15 ).
In rats, the involvement of taste receptor cells in the cephalic phase of digestion is well documented. The cephalic phase for insulin release (CPIR) in rats appears as early as 1–1·5 min after stimulation with saccharin or glucose( Reference Ionescu, Rohner-Jeanrenaud and Proietto 16 ). In humans, the presence of a CPIR and its physiological importance is somewhat controversial. ‘Tease feeding’ (exposure to sights and smells of a meal) or ingestion of the non-energetic sweetener aspartame individually fails to stimulate an early cephalic response for insulin. However, the combination of a tease meal plus aspartame elicits a drop in blood glucose levels and a rise in serum insulin and C-peptide levels within 5 min( Reference Bruce, Storlien and Furler 17 ). Other investigators have found that sweet taste stimuli provided as nutritive and non-nutritive sweetened tablets significantly decrease plasma glucose and insulin levels and that only sucrose causes plasma glucose and insulin levels to rise( Reference Abdallah, Chabert and Louis-Sylvestre 18 ). Furthermore, one set of experiments with nutritive and non-nutritive stimuli (aspartame, saccharin and sucrose) has shown that sweeteners in solutions do not provide adequate stimuli to elicit CPIR( Reference Teff, Devine and Engelman 19 ). However, others have found that sucrose and saccharin applied on the tongue elicit a significant increase in plasma insulin concentrations within 10 min, even when applied to the oral cavity only( Reference Just, Pau and Engel 20 ). Studies using functional MRI to monitor hypothalamic responses have shown that sweet taste (aspartame) or energy content (maltodextrin) individually does not elicit CPIR, but that together sweet taste and energy content (glucose) trigger CPIR( Reference Smeets, de Graaf and Stafleu 21 ).
We have found that in WT and Tas1r3KO mice local stimulation of taste cells of the tongue with glucose causes a significant increase in blood GLP-1 levels. The present results indicate that even after truncal vagotomy, which included oesophagectomy that would prevent any solution to enter the stomach, blood GLP-1 levels increased after oral stimulation with glucose. Thus, in mouse, there is the possibility that any early cephalic phase of GLP-1 release originates from the GLP-1-expressing taste cells of the tongue. This is an altogether novel basis for cephalic-phase hormone release for a hormone that was thought not to be released during the cephalic phase( Reference Ahrén and Holst 15 ). Because food and digestion products do not reach the intestines for several minutes after feeding, any early cephalic GLP-1 response could be mediated by release from endocrine taste cells and/or by efferent actions of the vagus nerve to stimulate intestinal enteroendocrine L cells to release GLP-1. It remains to be determined if such mechanisms hold true in humans.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513002262
Acknowledgements
The present study was supported by NIH grants DC03055, DC03155 and DK081421 to RFM. Imaging was performed at the Monell Histology and Cellular Localisation Core, which is supported in part by funding from NIH-NIDCD core grant 1P30DC011735-01. Z. K., K. K. Y., B. M. and R. F. M. designed the experiments; Z. K., K. K. Y., E. I., K. I. and Y. L. collected and analysed the data; all the authors contributed to the writing of the manuscript. The authors declare no conflicts of interest.










