Paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP) is the most frequent analgesic and antipyretic drug of widespread use in older persons. As APAP is the large-scale first-line treatment of chronic pain of low to moderate intensity, it is often used over repeated cure periods( Reference Airaksinen, Brox and Cedraschi 1 – Reference Makris, Abrams and Gurland 3 ). APAP detoxification initiates in the liver through phase I and II reactions( Reference Forrest, Clements and Prescott 4 , Reference Hodgman and Garrard 5 ). Up to 90 % of APAP is directly conjugated with sulphate (sulphation pathway) or glucuronide. In phase I, APAP is converted by cytochrome P450 into a highly reactive compound N-acetyl-p-benzoquinone imine (NAPQI). When the APAP dose increases, sulphation can become saturated then both glucuronide conjugation and, more significantly, oxidation to NAPQI, become higher. NAPQI is neutralised by GSH (γ-glutamyl–cysteinyl–glycine), and then metabolised through the mercapturic acid pathway. The end products of APAP detoxification processes are excreted along with urine. Sulphate and GSH being both issued from cysteine (Cys)( Reference Obled, Papet and Breuillé 6 ), APAP detoxification induces a net loss of this sulphur amino acid. Cys is provided by both dietary proteins and the breakdown of body proteins or GSH. Cys can also be endogenously synthesised from methionine and serine through the trans-sulphuration pathway. When the endogenous disposal of Cys is insufficient regarding its metabolic utilisations, Cys becomes an indispensable amino acid( Reference Obled, Papet and Breuillé 6 ). The net loss of sulphur to detoxify 3 g of APAP/d corresponded to 20 % of the sulphur amino acids ingested by older patients eating 1 g of proteins/kg body weight per d( Reference Pujos-Guillot, Pickering and Lyan 7 ). However, an arithmetic computation suggested that many APAP-treated older persons could be deficient in sulphur amino acids due to low dietary intakes( Reference Nimni, Han and Cordoba 8 ).
The adverse outcome of APAP on Cys/GSH homoeostasis is documented( Reference Reicks, Calvert and Hathcock 9 – Reference Mast, Savary-Auzeloux and Remond 11 ), and depletion of liver GSH is the key player leading to hepatotoxicity in case of over dosage( Reference Jaeschke 12 ). Within the therapeutic range, that is up to 4 g/d for humans, APAP is usually considered to be safe. However, a recent meta-analysis including eight observational studies highlighted increases in cardiovascular and gastrointestinal disorders and mortality with regular intake of therapeutic high doses( Reference Roberts, Delgado Nunes and Buckner 13 ). It is also known that some individuals are sensitive to mild liver injury at therapeutic doses( Reference Amar and Schiff 14 – Reference Winnike, Li and Wright 16 ). Several cases of severe APAP-induced hepatotoxicity have also been reported with therapeutic doses( Reference Eriksson, Broome and Kalin 17 – Reference Kurtovic and Riordan 19 ). These severe cases revealed a clear association of APAP-induced hepatotoxicity with fasting/undernutrition. This association was confirmed in an animal model mimicking undernourished patients, where protein–energy-restricted rats appeared to be more sensitive to APAP-induced hepatotoxicity than ad libitum fed rats( Reference Kondo, Yamada and Suzuki 20 ). These protein–energy-restricted rats also exhibited lower GSH levels under APAP, a key condition that can initiate hepatotoxicity( Reference Mitchell, Jollow and Potter 21 ). Knowing that Cys is the rate-limiting substrate for GSH synthesis( Reference Meister 22 ); it could be considered that protein–energy-restricted rats under APAP suffer from a shortage of Cys.
Based on the mechanism of APAP-induced hepatotoxicity, the pharmacological molecule N-acetylcysteine (NAC) is the standard antidote which should be given as early as possible after poisoning( Reference Hodgman and Garrard 5 , Reference Prescott, Park and Ballantyne 23 , Reference Lauterburg, Corcoran and Mitchell 24 ). The co-administration of APAP and NAC prevented toxicity associated with a single toxic dosing( Reference Owumi, Andrus and Herzenberg 25 ). Nevertheless, NAC was found to be less efficient than a mixture of amino acids (Cys, methionine and serine) to protect the liver against a single administration of APAP in miceReference Di Pierro and Rossoni 26 ). Moreover, NAC did not prevent liver toxicity from chronic low-dose plus subacute high-dose paracetamol exposure in young or old mice( Reference Kane, Huizer-Pajkos and Mach 27 ). Further research is therefore required into how to alleviate the adverse metabolic outcomes of chronic treatments or repeated cures with APAP, notably for older persons.
The requirement in sulphur amino acids could be unachieved in elderly people chronically treated with APAP due to the low amount of food ingested. A dietary supplementation with Cys, the amino acid lost to ensure APAP detoxification, would be the most logical personalised nutritional strategy against the adverse metabolic outcomes of chronic treatments or repeated cures with APAP. Indeed, the APAP metabolism leads to extensive use of Cys that is definitively lost in the urine, thereby diverting Cys from its physiological uses. The adverse metabolic outcomes are not limited to the liver as they may also concern skeletal muscles. We recently published that non-toxic APAP treatments which decreased Cys/GSH availability for skeletal muscle, led to decreased muscle mass in adult rats( Reference Mast, Joly and Savary-Auzloux 10 ) and worsened sarcopenia (muscle loss linked to ageing) in old rats with suboptimal food intake( Reference Mast, Savary-Auzeloux and Remond 11 ). It is well known that if availability of one amino acid alone is limited or its metabolic need is increased (e.g. Cys in sepsis or acute inflammation), protein synthesis is compromised especially in muscle( Reference Obled, Papet and Breuillé 28 ). Direct provision of Cys and its indirect supplies through cystine, GSH and other peptides containing Cys are indispensable for the muscle. In fact, Cys cannot be synthetised within the muscle because it lacks the enzymes necessary to synthetise Cys from methionine( Reference Mudd, Finkelstein and Irreverre 29 – Reference Stipanuk and Ueki 31 ). Dietary Cys supplementation should allow Cys/GSH homoeostasis to be maintained, thus avoiding any Cys shortage for muscles and therefore any adverse effects of APAP on skeletal muscle. Of note, increasing Cys content in the diet successfully restored liver GSH pools( Reference Breuillé, Pouyet and Malmezat 32 ) and limited weight loss and muscle wasting( Reference Breuillé, Béchereau and Buffière 33 ) in septic rats. Dietary Cys supplementation has also been proven efficient in increasing (albeit weakly) Cys and GSH pools in ageing rats( Reference Vidal, Breuille and Serrant 34 ). Therefore, the objective of the study was to evaluate whether dietary Cys supplementation could prevent the adverse outcomes of repeated APAP cures on Cys/GSH homoeostasis in old rats with suboptimal food intake, and consequently preserve their skeletal muscles.
Methods
Animals and experimental design
This study was performed in accordance with the current legislation on animal care and experimentation in France and received the approval (CE 08-13) of the local Ethical Committee, Comité d’Ethique en Matière d’Expérimentation Animale Auvergne. Male 20-21-month-old Wistar rats (Janvier Labs) were acclimatised 4 weeks before treatment in individual cages under standard conditions (22±1°C, 12 h light–12 h dark cycle) with free access to water and the control diet (Table 1). At the end of this adaptation period, the rats were divided into three groups based on weight, food consumption and body composition. Body composition was assessed using MRI (Echo MRI International). In all, thirty-six rats (APAP group) were submitted to three cures (C1 to C3) of 2 weeks of APAP treatment, intercalated with washout periods (inter-cure, IC) of 2 weeks (Table 2). As previously explained, ‘the cure model was chosen to reproduce the treatment of chronic pain in humans defined as daily pain lasting for at least 3 months( Reference Ferrell, Argoff and Epplin 35 ) and the alternation of painful and remission periods in patients. The length of 2 weeks for cures and IC took into account the difference between rat and human life expectancies’( Reference Mast, Savary-Auzeloux and Remond 11 ). In all, thirty-seven rats (APAP–Cys group) received the APAP–Cys diet instead of the APAP diet. The control group (CT, n 36) received the control diet throughout the experimental period. The APAP–Cys and CT groups received a quantity of food adjusted to the real consumption of the APAP group throughout the experimental period. Food consumption was recorded daily and body weight twice a week.
Table 1 Composition of the experimental dietsFootnote *
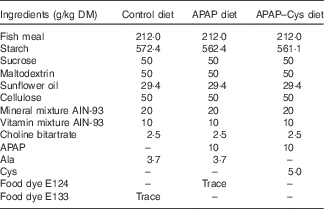
APAP, paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide); APAP–Cys, paracetamol–cysteine.
* The composition of the control diet was 16 % proteins, 68 % carbohydrates, 6 % fat, 5 % fibre and 5 % others.
Table 2 Distribution of the diets over the experimental periods according to the experimental groupFootnote *
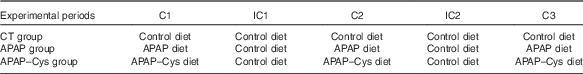
C, cure; IC, inter-cure; CT, control; APAP, paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide); APAP–Cys, paracetamol–cysteine.
* APAP–Cys and CT groups were pair-fed to the APAP group. Each C or IC lasted 2 weeks.
Diets
Repeated APAP cures were performed as previously described, the APAP powder (Sigma-Aldrich) being mixed with other ingredients( Reference Mast, Savary-Auzeloux and Remond 11 ) (Table 1). Food dyes were added to diets for identification purposes and to avoid any confusion during feeding periods. The dietary level of APAP (1 % w/w) was chosen as an equivalent dose of 4 g/d for humans( Reference Mast, Joly and Savary-Auzloux 10 ). Consumption of the 1 % APAP diet was expected to provide a daily dose within the range of the typical anti-nociceptive doses (200–300 mg/kg) for rats( Reference Muth-Selbach, Tegeder and Brune 36 ). l-Cys (Sigma-Aldrich) was also mixed with the ingredients to prepare the APAP–Cys diet. The Cys supplement was calculated to compensate for APAP-induced sulphur urinary loss on the basis that 54 % of APAP molecules was conjugated with sulphate or GSH in our previous study( Reference Mast, Joly and Savary-Auzloux 10 ). Taking into account molar masses of APAP and Cys, the 1 % APAP diet was supplemented with 0·5 % (w/w) of l-Cys. The equivalent Cys dosage for humans would be about 2 g/d, an intake within the nutritional range, thus expected to be safe. The APAP and control diets were supplemented with l-alanine to make them iso-nitrogenous to the APAP–Cys diet.
In vivo protein synthesis
Rates of protein synthesis were measured in the liver and muscle at the end of the third cure using the flooding dose method as previously described( Reference Mast, Savary-Auzeloux and Remond 11 ). After an overnight fast, [1-13C]valine (Cambridge Isotope Laboratories) (98·6 %, 150 μmoles/100 g body weight) was injected into a lateral tail vein 25 min before euthanasia (50 mg pentobarbital/kg body weight, intra peritoneal) to flood the precursor pool for protein synthesis. Blood was sampled from the aorta, plasma was separated by centrifugation at 2000 g for 15 min at 4°C, and immediately frozen in liquid N2. The liver was immediately removed, washed with saline and weighed. Skeletal muscles: gastrocnemius (GM), tibialis anterior (TA), soleus (SOL) and extensor digitorum longus (EDL) were carefully dissected from the left posterior leg and weighed. Immediately after weighing, the liver and GM were frozen in liquid N2. The GM was chosen for protein synthesis measurements because of its mixed-to-slow fibre type and its size allowing multiple assays once milled. All frozen samples were stored at −80°C before analyses. Frozen tissues were finely pulverised in liquid N2 using a ball mill (Dangoumeau) before analyses.
Free and protein-bound valine enrichments were determined by mass spectrometer and protein quantified using the bicinchoninic acid method, as previously described( Reference Savary-Auzeloux, Magne and Migné 37 ). Fractional and absolute synthesis rates were calculated, as previously described( Reference Mast, Savary-Auzeloux and Remond 11 ).
GSH and other aminothiols
Total free GSH (GSH, GSSG and other small disulphides) concentration was quantified in the liver, GM and blood with an automated analyzer (ABX Pentra 400; Horiba) using a standard enzymatic recycling procedure and 5,5'- dithio-bis-2-nitrobenzoic acid (Ellman reagent) as oxidant, as previously described( Reference Malmezat, Breuillé and Pouyet 38 ).
Plasma concentrations of free and protein-bound aminothiols (Cys, GSH, γ-glutamyl–cysteine (γ-Glu–Cys), cysteinyl–glycine (Cys–gly) and homocysteine (Hcy) were quantified by reversed-phase HPLC, as previously described( Reference Mast, Savary-Auzeloux and Remond 11 ). In fact, protein-bound γ-Glu–Cys was too low to be accurately assessed.
Hepatotoxicity and inflammatory markers
Hepatotoxicity was assessed by measurement of plasma alanine transaminase (ALT) and aspartate transaminase (AST) activities by photometry using an automated analyzer (ABX Pentra 400) and test kits A11A01629 and A11A01627 (Horiba), respectively. Plasma acute phase protein (α 2-macroglobulin, fibrinogen and albumin) concentrations were quantified as previously described( Reference Mayot, Vidal and Martin 39 ).
Statistical analysis
Group size was based on our previous results( Reference Mast, Savary-Auzeloux and Remond 11 ) to allow for the detection of differences for GM mass with a sufficient power of 80 % at the level of significance of 0·05. Results are expressed as means with their standard errors. Food intake and body weight were analysed using ANOVA for repeated measures with time as the within-rat factor and group as the second variable. The significance of differences was further analysed by Ryan–Einot–Gabriel–Welch q (REGWQ) test. Endpoint results were analysed using the one-way ANOVA, followed by the Tukey test. Analyses were performed using XLSTAT for Windows, version 2013.1.01 software (Addinsoft) and the significance was set at P≤0·05.
Results
Daily food intake and body weight
During the adaptation period, there was no significant difference in the food intake and body weight between groups (Table 3). Whatever the experimental period, food intakes were lower than during the adaptation period. As the CT and APAP–Cys groups were pair-fed to the APAP group, daily food intake of the three groups matched perfectly during cures and IC (no group effect or interaction with time effect). The daily APAP consumption during the cures was about 300 mg/kg per d. Body weight decreased throughout the experiment in all groups with no group effect, the overall body weight loss being about 7 % (Table 3).
Table 3 Food intake, body weight and lean mass over the experimental periods in control (CT), paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP) and paracetamol–cysteine (APAP–Cys) treated groupsFootnote * (Mean values with their standard errors)
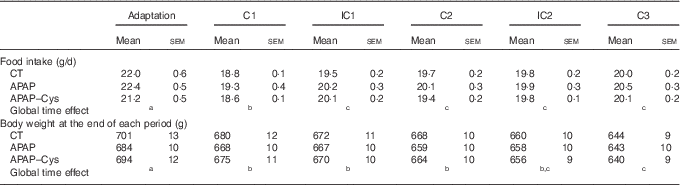
C, cure; IC, inter-cure.
a,b,c Values with unlike superscript letters were significantly different (P<0·05) within time points (REGWQ test).
* ANOVA for repeated measurements with time as the within-rat factor and group as the second variable for food intake: time: P<0·0001, group: P=0·120, time×group: P=0·293; body weight: time: P<0·0001, group: P=0·869, time×group: P<0·001.
Tissue GSH
Total free GSH concentration in the liver at the end of the experiment was 14 % lower in the APAP group compared with the CT group (Fig. 1(A)). Liver GSH was higher in the APAP–Cys group than the APAP and CT groups by 35 and 15 %, respectively. There was no significant difference in GM concentration in total GSH between the three groups (Fig. 1(B)). Total blood GSH concentration was 67 % higher in the APAP group than the CT group. The addition of Cys to APAP cures normalised blood GSH (Fig. 1(C)).
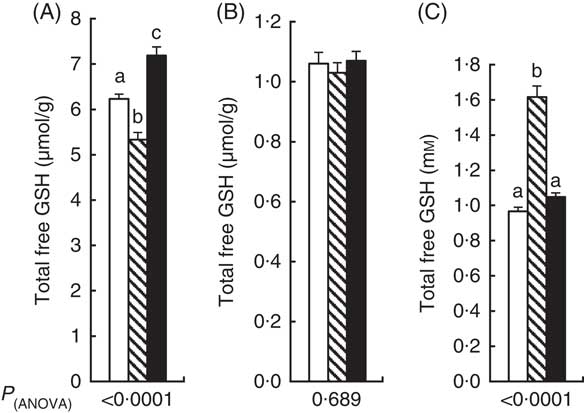
Fig. 1 Total free GSH (GSH, GSSG and other small disulphides) concentration in liver (A), gastrocnemius muscle (B) and blood (C) of control, paracetamol and paracetamol–cysteine treated groups. ![]() , control group;
, control group; ![]() , paracetamol group (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP);
, paracetamol group (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP); ![]() , APAP–cysteine group. a,b,c Mean values with unlike letters were significantly different (ANOVA followed by the Tukey test, P<0·05).
, APAP–cysteine group. a,b,c Mean values with unlike letters were significantly different (ANOVA followed by the Tukey test, P<0·05).
Plasma aminothiols
Compared with CT, the plasma concentration of free Cys (Cys+cystine+small Cys disulphides) decreased by 10 % with repeated cures with APAP alone (Fig. 2(A)). Plasma free Cys of the APAP–Cys group did not differ significantly from the other two groups. There was no significant difference in plasma concentrations of the other free aminothiols (GSH, γ-Glu–Cys, Cys–Gly and Hcy) between the three groups. Compared with CT, the plasma concentrations of protein-bound Cys–Gly and Hcy were 18 and 27 % higher in the APAP group than the CT group, respectively (Fig. 2(B)). Plasma protein-bound Cys–Gly and Hcy of the APAP–Cys group did not differ significantly from the other two groups. There was no significant difference in plasma concentrations of protein-bound Cys and GSH between the three groups.
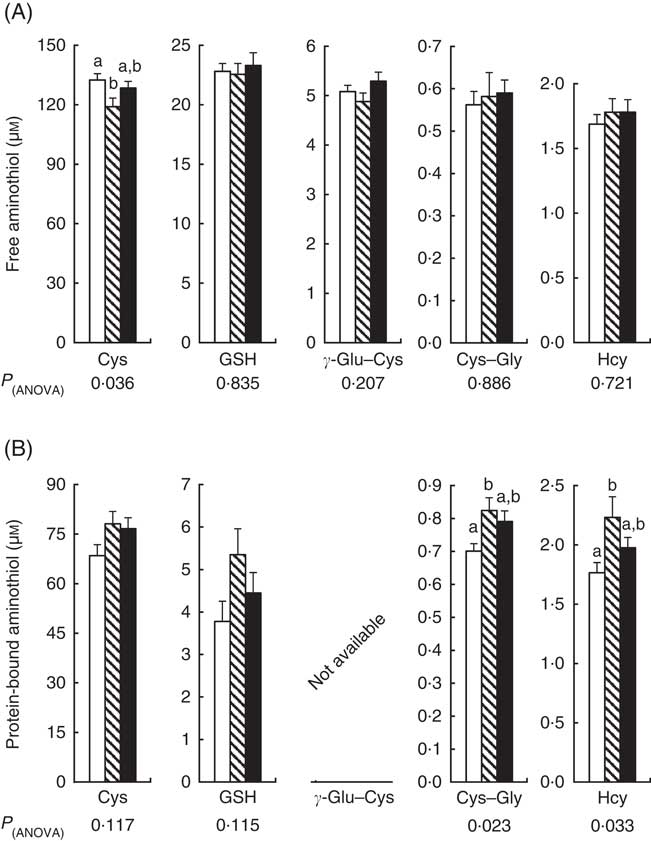
Fig. 2 Plasma free (A) and protein-bound (B) aminothiols in control, paracetamol and paracetamol–cysteine treated groups. ![]() , control group;
, control group; ![]() , paracetamol group (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP);
, paracetamol group (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP); ![]() , APAP–cysteine (Cys) group; Cys, cysteine; Cys–gly, cysteinyl–glycine; γ-Glu–Cys, γ-glutamyl–cysteine; Hcy, homocysteine. a,b Mean values with unlike letters were significantly different (ANOVA followed by the Tukey test, P<0·05).
, APAP–cysteine (Cys) group; Cys, cysteine; Cys–gly, cysteinyl–glycine; γ-Glu–Cys, γ-glutamyl–cysteine; Hcy, homocysteine. a,b Mean values with unlike letters were significantly different (ANOVA followed by the Tukey test, P<0·05).
Tissue masses, protein contents and synthesis rates
Liver mass was 15 % higher in the APAP group compared with the CT group (Table 4). The addition of Cys to APAP cures normalised the liver mass. There was no significant difference in the protein contents of the liver between the three groups (Fig. 3(A)). Amongst the three groups, there was only a difference in the fractional synthesis rate of liver proteins between APAP–Cys and APAP groups, with an 8 % decrease induced by the addition of Cys to the APAP cures (Fig. 3(A)). Despite a significant group effect on the absolute synthesis rate of liver proteins, post-ANOVA tests reveal no significant effect between groups when compared with each other (Fig. 3(A)).
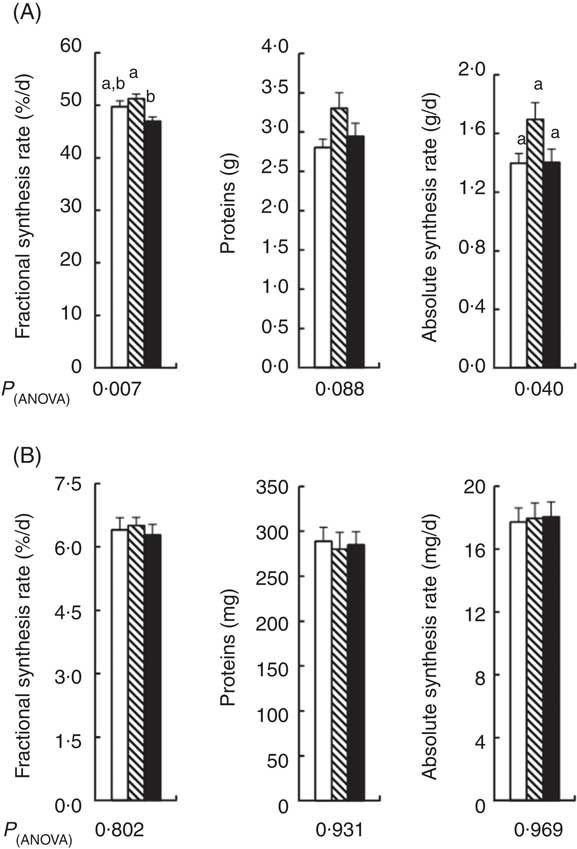
Fig. 3 Fractional synthesis rate, protein content and absolute synthesis rate in the liver (A) and gastrocnemius muscle (B) of control (![]() ), paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP,
), paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP, ![]() ) and paracetamol–cysteine (APAP–Cys,
) and paracetamol–cysteine (APAP–Cys, ![]() ) treated groups. a,b Mean values with unlike letters were significantly different (ANOVA followed by the Tukey test, P<0·05).
) treated groups. a,b Mean values with unlike letters were significantly different (ANOVA followed by the Tukey test, P<0·05).
Table 4 Liver and muscle masses in control (CT), paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP) and paracetamol–cysteine (APAP–Cys) treated groups (Mean values with their standard errors)
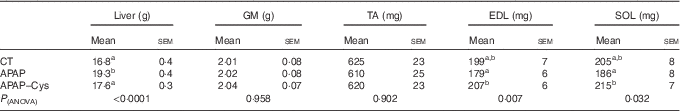
GM, gastrocnemius; TA, tibialis anterior; EDL, extensor digitorum longus; SOL, soleus.
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·05, ANOVA followed by the Tukey test).
There was no significant difference in masses of GM and TA between the three groups and the masses of EDL and SOL of the CT group did not significantly differ from the APAP and APAP–Cys groups (Table 4). However, the addition of Cys to APAP cures increased EDL and SOL masses by 16 % each. There was no significant difference in the protein content of GM or the fractional and absolute synthesis rates of GM proteins between the three groups (Fig. 3(B)).
Hepatotoxicity and inflammation
Amongst the three groups, the activity of ALT only differed between the APAP–Cys and APAP groups; with a 30 % decrease induced by the addition of Cys to the APAP cures (Table 5). There was no significant difference in plasma AST activity or plasma concentrations of α 2-macroglobulin and fibrinogen between the three groups (Table 5). Plasma albumin concentration was 13 % higher in the APAP–Cys group than the CT group (Table 5).
Table 5 Transaminase activities and acute phase proteins in the plasma of control (CT), paracetamol (acetaminophen, N-acetyl-para-aminophenol, 4-hydroxy-acetanilide, APAP) and paracetamol–cysteine (APAP–Cys) treated groups (Mean values with their standard errors)

ALT, alanine transaminase; AST, aspartate transaminase.
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·05, ANOVA followed by the Tukey test).
Discussion
Adverse metabolic outcomes occur in old rats submitted to repeated cures with a 1 % APAP diet( Reference Mast, Savary-Auzeloux and Remond 11 ). In the present experiment, the addition of Cys to the APAP cures, at a level equivalent to the detoxification needs, was efficient to prevent all APAP-induced adverse outcomes for the liver where detoxification takes place, but also blood GSH, and to some extent, plasma free Cys and protein-bound Cys–Gly and Hcy. These results were obtained in old rats with suboptimal food intake under repeated cures with a non-toxic APAP dosage an animal model of APAP-treated older persons.
Liver GSH data extend previous observations made by others in growing or adult mice under chronic treatment with APAP( Reference Reicks, Calvert and Hathcock 9 , Reference Reicks and Hathcock 40 ). In these studies, providing methionine largely above the requirement level (up to 1 % of the diet (w/w) for methionine as well as for APAP) prevented APAP-induced decreases in hepatic GSH. Cys supplementation is presently efficient, whereas methionine is not synthesisable from Cys. Thus, the beneficial effect of methionine on liver GSH is likely to have resulted from its capacity to provide Cys, meaning that the endogenous synthesis of Cys from methionine and serine was insufficient to maintain liver GSH when APAP-treated animals received a standard diet. Methionine is the indispensable sulphur amino acid, whose overload may have adverse cardiovascular effects directly or through an accumulation of Hcy( Reference Osborne-Pellegrin and Fau 41 , Reference Hannibal and Blom 42 ). Cys is a conditionally indispensable amino acid because its endogenous synthesis can become insufficient to meet its metabolic utilisations in some circumstances( Reference Obled, Papet and Breuillé 6 ). Altogether, the requirement of sulphur amino acids is increased by APAP and Cys can fulfil this demand.
The paradoxical large APAP-induced increase in blood GSH concentration confirms our previous observation in adult rats treated for 17 d with APAP( Reference Mast, Joly and Savary-Auzloux 10 ). The GSH concentration in blood basically accounts for its concentration in erythrocytes, blood concentration being about 40-fold that of plasma. The GSH concentration in erythrocytes depends only on the balance between its intracellular synthesis and the export of its oxidised form, as there are no known degradative pathways for GSH in erythrocytes and no efficient transport of the reduced form of GSH across their membrane( Reference Srivastava 43 ). Whatever the mechanism involved in the APAP-induced increase in blood GSH, the most interesting observation is its normalisation by Cys supplementation meaning that Cys deficiency plays a key role in APAP-induced increase in blood GSH. This is in agreement with the increased blood GSH already reported in methionine-restricted rats, also associated with decreases of liver GSH and plasma free Cys( Reference Richie, Leutzinger and Parthasarathy 44 , Reference Richie, Komninou and Leutzinger 45 ). The increase in blood GSH appears to reflect the deficiency in Cys observed in APAP-treated rats. Thus, blood GSH may be helpful to determine the optimum Cys supplementation needed to prevent APAP-induced adverse metabolic outcomes.
APAP repeated cures decreased plasma protein-bound Cys–Gly and Hcy, and Cys supplementation compensated these effects to some extent. Aminothiols are either free or bound to proteins through disulphur bridges. Any increase in protein-bound forms without any modification in free forms reveals a modification of the redox status toward a more oxidative state. Thus, Cys supplementation prevented the pro-oxidative effect of repeated APAP cures. It also increased plasma albumin v. the CT group. As oxidative stress has been implicated in the pathogenesis of several ageing-associated pathologies( Reference Manoharan and Guillemin 46 , Reference Gomes, Martinez and Pagan 47 ) and hypoalbuminemia is a mortality prognostic factor in elderly people( Reference Cabrerizo, Cuadras and Gomez-Busto 48 ), it could be considered that Cys supplementation could have a real beneficial effect in elderly persons under chronic/repeated cures with APAP.
Muscle mass was not significantly reduced by repeated APAP cures, contrasting with our previous experiment performed with the same experimental design( Reference Mast, Savary-Auzeloux and Remond 11 ). The difference in the susceptibility of old rats to APAP-induced negative effects between our two studies performed with the same experimental design could be attributed to the well-known inherent high variability between the cohorts of old rats( Reference Ghirardi, Cozzolino and Guaraldi 49 ). That difference could not be attributed to a lower APAP dose really ingested by old rats as it reached 300 mg/kg per d, a dose equivalent to a daily therapeutic dose of 3·5 g/d for humans, whereas the APAP dose amounted to 260 mg/kg per d in the previous experiment( Reference Mast, Savary-Auzeloux and Remond 11 ). In spite of a higher dose of APAP in the present study, no hepatotoxicity was recorded. Indeed, the present low variation in ALT is definitively below the threshold levels usually considered for hepatotoxicity, that is, three times the control values of AST and ALT( Reference Temple 50 ). Plasma concentrations of acute phase proteins revealed no APAP-induced inflammation. Consistently with these two observations, the low impact on protein synthesis in the liver, avoids competition between liver and muscle for the use of Cys. The main difference between our two studies was a food intake under APAP cures 12 % higher in the second experiment than the first one( Reference Mast, Savary-Auzeloux and Remond 11 ). This difference may explain why APAP-induced alterations in liver GSH, plasma free Cys (also named cyst(e)ine) and protein-bound Cys–gly and Hcy were presently light. Most importantly, these variations were not accompanied by a decline in plasma free GSH, whereas plasma free GSH was previously decreased with APAP( Reference Mast, Savary-Auzeloux and Remond 11 ). The milder effect of APAP repeated cures on plasma free Cys associated with the absence of effect on plasma free GSH, a supplier of Cys, means that the decrease in the availability of Cys for the peripheral tissues, such as muscle, was definitively weak in the present study. It is likely that the present mild alteration in peripheral Cys/GSH homoeostasis was not sufficient to significantly affect muscle GSH and proteins. Altogether, the adverse metabolic outcomes induced by APAP in the liver and plasma appear to be milder when food intake is higher. These mild alterations were insufficient to impair muscle.
A typical design with APAP and Cys as experimental factors would have included a group of rats receiving the Cys supplementation without APAP and pair-fed to the three other experimental groups. In our previous experiment, a similar Cys supplementation induced only minor increases in Cys and GSH and no effect on muscle masses in old rats fed ad libitum ( Reference Vidal, Breuille and Serrant 34 ). Thus, the conclusion of the present study would likely to have been unchanged by the inclusion of a group with a supplementation with Cys alone. Another limitation is that redox potentials (Eh) of the Cys/cystine and GSH/GSSG couples were not determined due to the technical choice to measure the total forms and the protein-bound forms and to calculate the free forms. However, knowing that the oxidised free form of GSH is always largely lower than its reduced form, the large present variations of either total or free forms can be confidently interpreted as significant variations in the reduced antioxidant form of GSH. In the same line of thought, oxidative stress was not deeply investigated in the present study because it is common knowledge that APAP treatment can induce oxidative stress consecutively to APAP-induced GSH decrease( Reference Jaeschke, McGill and Ramachandran 51 ). Thus, the present improvement in Cys/GSH homoeostasis in the APAP–Cys group can be considered with some certainty as a beneficial effect regarding oxidative stress.
In conclusion, sulphur amino acid requirement is increased by APAP and Cys can fulfil this demand. Indeed, dietary supplementation with Cys was efficient to improve Cys/GSH homoeostasis of old rats under repeated APAP cures. Cys supplementation exerted other beneficial effects to the liver and muscles that could have healthy outcomes. Moreover, Cys supplementation may be of further interest: (i) when a strong reduction of the peripheral availability of Cys/GSH occurs along with a generalised loss of muscle and (ii) for individuals susceptible to hepatotoxicity at therapeutic doses( Reference Amar and Schiff 14 – Reference Winnike, Li and Wright 16 ). Finally, dietary Cys supplementation could be beneficial to the health of older persons under APAP treatment due to frequent low food intakes( Reference Calvani, Miccheli and Landi 52 ) and the fact that their requirements in sulphur amino acids seem to be already higher than those of adults( Reference Tuttle, Bassett and Griffith 53 – Reference McCarty and DiNicolantonio 55 ).
Acknowledgements
The authors would like to acknowledge Medhi Djelloul-Mazouz, and Philippe Denis from the Installation Expérimentale de Nutrition for animal care.
This work was supported by the Institut National de la Recherche Agronomique, France and a Société francophone de nutrition clinique et métabolisme (SFNEP) – Antadir grant. SFNEP and Antadir had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: I. P. and C. Mast designed the research; C. Mast, C. P. G. V., C. Migné and D. C. performed the experiment, C. Mast, C. P. and I. P. analysed the results, C. Mast and I. P. wrote the manuscript; I. S-A., D. R. and D. D. revised the manuscript; I. P. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.













