There is evidence that excessive Na intake is associated with the development of hypertension and CVD in adults( Reference Kotchen, Cowley and Frohlich 1 ), and that modest reductions in Na intake can lead to a decrease in blood pressure in children( Reference He and MacGregor 2 ). Recent studies have also shown that Na intake is positively related to obesity in adults( Reference Ma, He and MacGregor 3 ) and children( Reference Grimes, Riddell and Campbell 4 ), independent of energy intake. In most developed countries, adults consume too much Na and this has also been observed in children, with intakes far exceeding dietary recommendations( Reference Brown, Tzoulaki and Candeias 5 ). K is a nutrient known to have beneficial effects on health in adults, including reducing blood pressure and risk of stroke and CVD, reducing age-related bone loss and reduction of kidney stones( Reference Weaver 6 ).
It has been suggested that a high-Na diet may lead to the development of chronic diseases via increased production of cortisol as measured in urine( Reference Baudrand, Campino and Carvajal 7 ). Cortisol, the main glucocorticoid in humans, is an important steroid hormone in the regulation of metabolism and stress, which is produced by activation of the hypothalamic–pituitary–adrenal axis( Reference Folkow 8 ). Cortisol can also be produced in tissues by the conversion of inactive cortisone to active cortisol where it can have direct biological effects at a local level( Reference Tomlinson and Stewart 9 ). Elevated cortisol has been linked to the development of numerous diseases in adults, including CVD, type 2 diabetes and the metabolic syndrome( Reference Chrousos 10 ). In a large cross-sectional study of adult men and women, hair cortisol, as a marker of long-term cortisol exposure, was positively associated with weight, BMI and waist circumference, and in a retrospective longitudinal analysis was found to be associated with obesity over 4 years( Reference Jackson, Kirschbaum and Steptoe 11 ). The potential detrimental effects of excess cortisol levels have also been observed in paediatric populations. For example, greater increases in salivary cortisol in response to mild psychological stress in children was associated with adverse effects on memory function( Reference Heffelfinger and Newcomer 12 ). Recent evidence shows that cortisol is elevated in children and adolescents with depression( Reference Stetler and Miller 13 ) and in obese prepubertal girls( Reference Papafotiou, Christaki and van den Akker 14 ).
There is evidence in adults to suggest that Na intake is positively associated with urinary cortisol concentrations. For example, in experimental studies conducted in adults, urinary free cortisol increases after consuming high-Na diets of 320 mEq/d( Reference Wambach, Bleienheuft and Bonner 15 ) and 200 mmol/d( Reference Litchfield, Hunt and Jeunemaitre 16 ) and decreases after restricting Na intake to 20( Reference Lewicka, Nowicki and Vecsei 17 ) and 10 mmol/d( Reference Chamarthi, Kolatkar and Hunt 18 ). While one study found no evidence of an association between 24 h urinary Na and cortisol levels, this study measured cortisol levels only in serum rather than urine( Reference Afsar and Ay 19 ). These findings are consistent with those of Baudrand et al.( Reference Baudrand, Campino and Carvajal 7 ) who measured cortisol in both plasma and urine. These investigators showed that urinary free cortisol and total cortisol metabolite levels in urine, but not plasma, were significantly higher in participants with high Na intake compared with those with an adequate Na intake( Reference Baudrand, Campino and Carvajal 7 ). These latter two studies suggest that it is not sufficient to measure cortisol in plasma/serum as these levels remain well controlled within a stable homoeostatic range. Instead, it appears necessary to measure cortisol levels in urine. While the levels of cortisol in plasma would intuitively seem to be the most important, as these are what the tissues are exposed to, when cortisol are produced within tissues, it can have detrimental effects at a local level before entering the systemic circulation and being cleared rapidly by the kidney. Measures of both urinary free cortisol and total cortisol metabolites are necessary to provide a complete picture of urine cortisol excretion, which is thought to be indicative of overall daily cortisol production( Reference Baudrand, Campino and Carvajal 7 ). Although there is evidence of an association between Na intake and urinary cortisol in adults, no studies have examined this association in children.
Thus, the primary objective of this study was to test the hypothesis that 24 h urinary Na is positively associated with 24 h urinary free cortisol and total cortisol metabolites in a sample of Australian schoolchildren aged 5–12 years and their mothers. Given the known health benefits of higher K intake, and the lack of data available on K and cortisol in adults and children, we further hypothesised that children and their mothers would show a negative association between 24 h urinary K and urinary free cortisol and total cortisol metabolites. This was part of a larger cross-sectional study in which we have previously reported a positive association between Na intake and obesity risk in Australian schoolchildren( Reference Grimes, Riddell and Campbell 4 ).
Methods
Study design and participants
In this study, we measured urinary free cortisol and cortisol metabolites in 24 h urine samples in a sub-group of primary schoolchildren and their mothers who had participated in the Salt and Other Nutrient Intakes in Children (SONIC) study, which included children and their parents sampled from primary schools located in Victoria, Australia from 2009 to 2013. A detailed description of the study protocol and the primary results have been reported previously( Reference Grimes, Riddell and Campbell 20 , Reference Grimes, Baxter and Campbell 21 ). The initial sample size for this study was determined by the number of mothers with available Na data, which was 107 mothers. If two or more children from one family had participated in the SONIC study, we randomly selected one child per mother to be included. A total of five mothers were subsequently excluded because cortisol data could not be obtained, but we chose to keep their child in the present study. As we wished to look at the association between urinary Na and urinary cortisol across the widest possible range of Na intakes in children, we included an additional seven children with Na levels (249–310 mmol/24 h) higher than the range of the already selected children (31–245 mmol/24 h) and six children with Na levels lower than this selected range (13–30 mmol/24 h). Therefore, our sample included 102 mother–child pairs and a total of 120 children.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki. Mothers and children provided written informed consent before commencing the study, which was approved by the Deakin University Human Ethics Committee (Project no. EC 62-2009).
Measures
Socio-economic status (SES) was based on school postcode, as previously defined( Reference Grimes, Riddell and Campbell 4 ).
24 h urine collection
The 24 h urine collection protocol has been described elsewhere( Reference Grimes, Riddell and Campbell 4 , Reference Grimes, Baxter and Campbell 21 ). In brief, at the start of the 24 h urine collection, children and mothers were instructed to empty their bladder, discard this urine and note this as the start time of their collection. Following this, all urine voided was collected until the 24 h finish time. Urine samples were considered incomplete and not included in the final analysis if collection time was <20 or >28 h, total volume was <300 ml, the participant reported missing >1 collection, or urinary creatinine for children were <0·1 mmol/kg body weight per d( Reference Grimes, Baxter and Campbell 21 ). Mothers with suspected inaccurate urine collections (i.e. urinary creatinine <4 mmol/d for women, or a 24 h urine collection of <500 ml) were excluded.
Aliquots of urine (2 ml) that were stored at –80°C were thawed under room temperature and utilised for free cortisol and cortisol metabolite analyses.
Urinary free cortisol analysis
Concentrations of urinary free cortisol were measured using an ELISA (Diametra). A total of seven assays were conducted with the lowest detection sensitivity of 2·95 ng/ml at the 95 % confidence limit. The intra-assay CV was 4·8 % at 47 ng/ml and 8·0 % at 287 ng/ml. The inter-assay CV was 10·8 % at 47 ng/ml and 7·9 % at 287 ng/ml.
Extraction of steroids and measurement of cortisol metabolites by GC–MS
Steroids were extracted from urine samples and measured using GC–MS based on previously published methods( Reference Honour 22 ) with some modifications. Briefly, deuterated internal standards, tetrahydrocortisone-D5 and tetrahydrocortisol-D5 (Sigma Aldrich), were added to samples followed by hydrolysis, extraction and derivatisation steps described previously( Reference Honour 22 ). N-dried samples were reconstituted in cyclohexane/pyridine/hexamethyldisilazane (98:1:1) and transferred to vials for GC–MS.
The GC–MS system consisted of Agilent components: a 6890 GC, a 7683 automated sampler and a 5975C mass selective detector. A 30 m × 0·25 mm (inner diameter) × 0·25 μm capillary column (VF-Xms; Agilent Technologies) was used with helium as the carrier gas (flow rate 1·1 ml/min). The GC oven programme consisted of an initial hold temperature of 70°C for 1·5 min, a 25°C/min ramp to 220°C, a 2°C/min ramp to 276°C, a 25°C/min ramp to 320°C, and a final hold time for 3 min at 320°C. Data recording and analysis occurred using MSD Chemstation software (Agilent Technologies). Endogenous cortisol metabolites, α-cortolone, β-cortolone, α-cortol, β-cortol, tetrahydrocortisone, tetrahydrocortisol and allo-tetrahydrocortisol and deuterated internal standards were identified using selective ion monitoring. External standard curves were run daily for each of the target metabolites for purposes of relative quantification. Each metabolite was quantitatively corrected for its own or its closest-related deuterated internal standard. The total cortisol metabolites in each sample were calculated as the sum of each individual cortisol metabolite concentration, corrected for the total urine collection volume.
Urinary sodium and potassium analysis
Returned 24 h urine samples were transported to an accredited commercial pathology laboratory (Dorevitch Pathology) for analysis. Urinary Na and K concentrations were assessed using indirect ion selective electrodes, and urinary creatinine concentration was assessed using the Jaffe reaction( Reference Jaffe 23 ) on the Siemens Advia 2400 analyser (Siemens Healthcare). Approximately 90–95 % of ingested Na is excreted in urine( Reference Bates, Thurnham and Bingham 24 ). Because of the high recovery of Na in urine, 24 h urine is considered the ‘gold standard’ method to determine dietary Na intake( 25 ). The CV for Na and K was <1 % and for creatinine it was 3·25 %. If the duration of the collection was not exactly 24 h but within 20–28 h, urinary electrolytes, creatinine, urinary free cortisol, urinary cortisol metabolites and total volume were standardised to a 24 h period (i.e. (24 h/urine duration (h)) × urinary measure).
Anthropometric measures
Height and weight of children were measured by trained research staff following standard protocols( Reference Grimes, Riddell and Campbell 4 , Reference Grimes, Baxter and Campbell 21 ). BMI was calculated as weight (kg) divided by height (m) squared. BMI values were converted to age- and sex-adjusted BMI z scores using the 2000 US Centers for Disease Control and Prevention growth charts( Reference Cole, Flegal and Nicholls 26 , Reference Cole, Bellizzi and Flegal 27 ). No anthropometry data were available for the mothers.
Statistical analysis
Descriptive statistics (mean values and standard deviations or numbers and percentages) were calculated to describe the participant characteristics. Differences between children and mothers 24 h urinary electrolytes and 24 h urinary cortisol were assessed using the Mann–Whitney U test. Multilevel mixed-effects linear regression was used to determine the association between urinary Na or urinary K and urinary free cortisol or total cortisol metabolites. All models included a random intercept for school. In children, unadjusted models and models adjusting for age, sex, urinary Na or urinary K are presented. In an additional model, we also adjusted for BMI z-score because obesity may influence the association between urinary electrolytes and urinary cortisol. In mothers, unadjusted models and models adjusting for age, urinary Na or urinary K were fitted. In addition, the associations were tested between mothers and children for urinary electrolytes and urinary cortisol. Model residuals were assessed for normality and heteroscedasticity using Q–Q plots and plots of residuals against fitted values.
On the basis of prior research( Reference Baudrand, Campino and Carvajal 7 ), we estimated an effect size of r 2 0·17 for the relationship between urinary Na and urinary free cortisol and r 2 0·10 for the relationship between urinary Na and urinary cortisol metabolites. Power analyses conducted separately for children (n 120) and mothers (n 100) showed that we had over 90 % power to detect these effects, for α=0·05. All statistical analysis, including power analysis, was conducted using SPSS 24.0 for Windows (SPSS Inc.) and STATA SE, version 15 (1985–2017; StataCorp).
Results
Participants
Table 1 shows the demographic characteristics and 24 h urinary electrolytes and cortisol for children and mothers. There was one mother excluded based on an extremely low urinary Na concentration (10 mmol/24 h, >3sd below the mean) and one mother excluded with an extremely high urinary free cortisol concentration (529 mmol/24 h, >3 sd above the mean). The final sample consisted of 120 children and 100 mothers. There was no difference in age, sex, SES or BMI z-score between those children in this sub-study compared with those in the original SONIC study (data not shown, P > 0·05 for all)( Reference Grimes, Riddell and Campbell 4 ). Overall, 53 % of children were girls and had an average age of 9·2 years. In total, 13 % of children were either overweight or obese. With respect to Na intake in the children, urinary Na ranged from 13 to 310 mmol/d with a mean of 104 mmol/d. There were forty-five (38 %) children who were at or below the recommended dietary Na upper level and seventy-five (63 %) children who exceeded the upper level (upper level: 4–8 years 60 mmol/d, 9–13 years 86 mmol/d)( 28 ). The average age of mothers was 41·7 years. In mothers, urinary Na ranged from 47 to 237 mmol/d with a mean of 121 mmol/d. There were thirty-six (36 %) mothers who were at or below the recommended Na upper level for adults of 100 mmol/d)( 28 ) and sixty-four (64 %) mothers who exceeded the upper level. Compared with children, the mothers had significantly higher levels of urinary electrolytes, urinary free cortisol and total cortisol metabolites (Table 1).
Table 1 Descriptive characteristics of participants (Mean values and standard deviations; numbers and percentages)
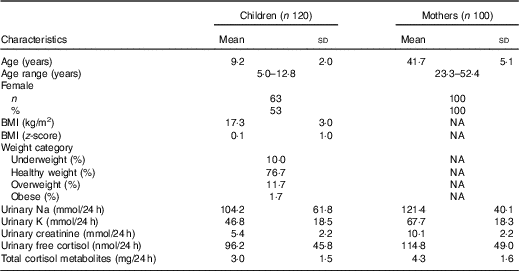
NA, not available.
* P < 0·05 (Mann–Whitney U test).
Associations between urinary electrolytes and urinary cortisol
Fig. 1 shows scatterplots for children’s 24 h urinary electrolytes and urinary cortisol and cortisol metabolites. Table 2 shows the multilevel mixed-effects linear regression models for children with urinary electrolytes as the independent variables and urinary free cortisol and urinary cortisol metabolites as the dependent variables. In children, urinary Na and K were both positively associated with urinary free cortisol and urinary cortisol metabolites, and this relationship persisted after adjusting for relevant confounders (age, sex, urinary Na, urinary K and BMI z-score). Results from the fully adjusted models show that a 1 mmol/24 h increase in urinary Na was related to a 0·31 (95 % CI 0·19, 0·44) nmol/24 h higher urinary free cortisol, and a 1 mmol/24 h increase in urinary K was related to a 0·65 (95 % CI 0·23, 1·07) nmol/24 h higher urinary free cortisol (Fig. 1). There was no evidence of an association between Na:K ratio and either urinary free cortisol or urinary cortisol metabolites (data not shown).
Table 2 The association between urinary electrolytes and urinary cortisol in schoolchildren and mothers (Regression coefficients and 95 % confidence intervals)
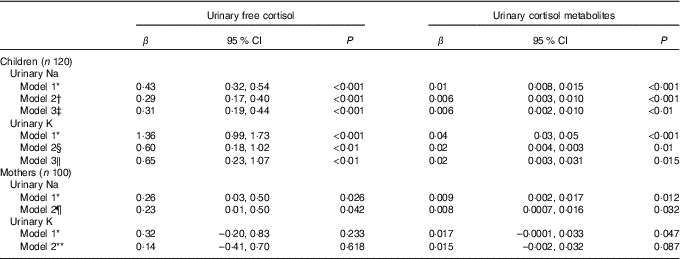
* Model 1: unadjusted.
† Model 2: adjusted for age, sex and urinary K.
‡ Model 3: adjusted for age, sex, urinary K and BMI z score.
§ Model 2: adjusted for age, sex and urinary Na.
‖ Model 3: adjusted for age, sex, urinary Na and BMI z score.
¶ Model 2: adjusted for age and urinary K (n 94 included in analysis).
** Model 2: adjusted for age and urinary Na (n 94 included in analysis).
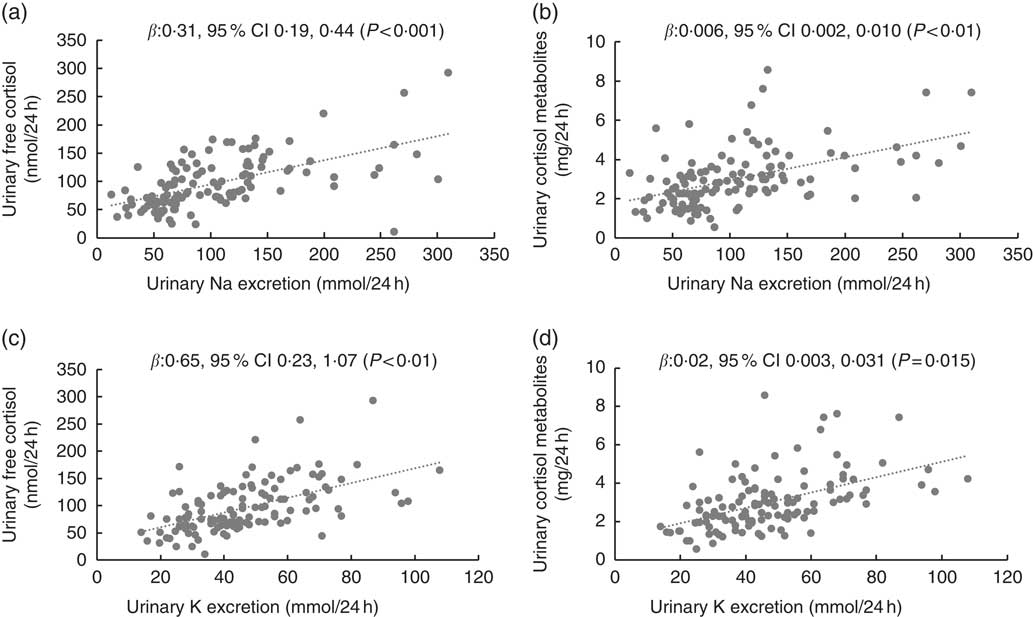
Fig. 1 Scatterplots for children’s 24 h urinary electrolytes and 24 h urinary cortisol (n 120). Scatterplots for (a) urinary sodium and urinary free cortisol, (b) urinary sodium and urinary cortisol metabolites, (c) urinary potassium and urinary free cortisol, (d) urinary potassium and urinary cortisol metabolites. Data were analysed with multilevel mixed-effects linear regressions adjusted for age, sex and BMI z score. In addition, adjusted for urinary potassium (a) and (b), and urinary sodium (c) and (d).
In mothers, urinary Na was positively associated with urinary free cortisol and cortisol metabolites in both the unadjusted and adjusted models (Table 2 and Fig. 2). Urinary K was positively associated with urinary cortisol metabolites in the unadjusted model but not in the adjusted model and urinary K was not associated with urinary free cortisol in unadjusted or adjusted models. In mothers, urinary Na was positively associated with urinary K (r 0·266, P < 0·05).
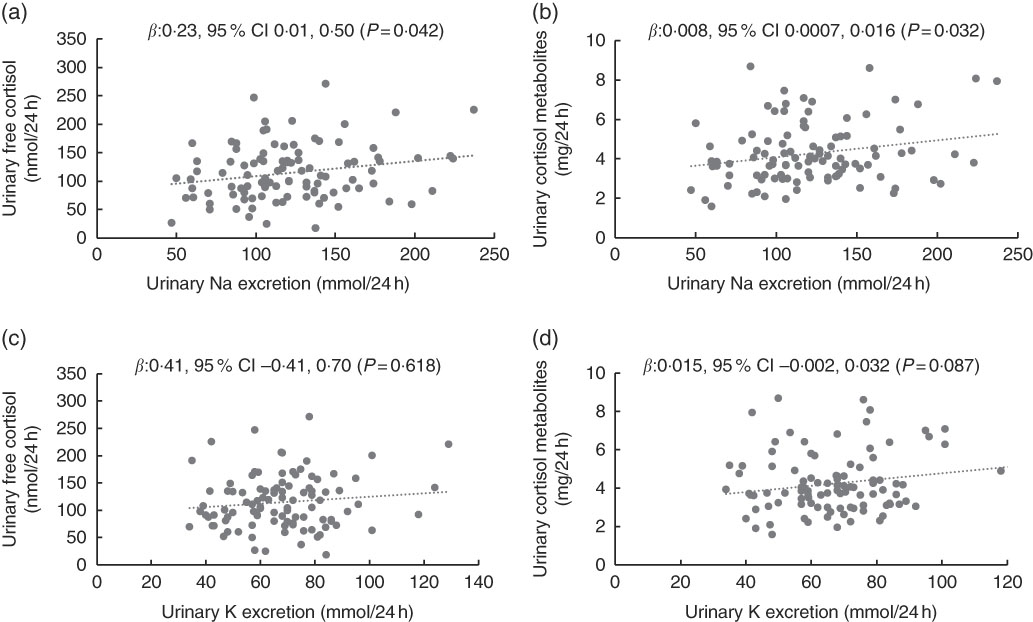
Fig. 2 Scatterplots for mother’s 24 h urinary electrolytes and 24 h urinary cortisol (n 100). Scatterplots for (a) urinary sodium and urinary free cortisol, (b) urinary sodium and urinary cortisol metabolites, (c) urinary potassium and urinary free cortisol, (d) urinary potassium and urinary cortisol metabolites. Data were analysed with multilevel mixed-effects linear regressions adjusted for age. In addition, adjusted for urinary potassium (a) and (b), and urinary sodium (c) and (d).
Within mother–child dyads, urinary Na, free cortisol and cortisol metabolites were positively associated (P < 0·05) between mothers and children’s, but urinary K was not (Table 3).
Table 3 Pairwise associations between urinary electrolytes and urinary cortisol in mother–child dyads (n 100) (Regression coefficients and 95 % confidence intervals)

Discussion
Among a sample of Australian schoolchildren, we have reported novel data for the first time that higher 24 h urinary Na and K levels were associated with higher 24 h urinary free cortisol and urinary cortisol metabolites. In mothers, urinary Na was positively associated with both urinary free cortisol and cortisol metabolites, while urinary K was not associated with urinary free cortisol or cortisol metabolites. Associations in children remained after adjusting for age, sex, BMI z score, and urinary Na or urinary K. Overall, the magnitude of the associations was greatest in the children. We also found that urinary Na, free cortisol and cortisol metabolites were positively associated between children and mothers. It should be noted that causation cannot be established due to the cross-sectional nature of this study.
The level of urinary free cortisol in the children in the present study was similar to that in a population of obese children( Reference Reinehr, Kulle and Wolters 29 ), but higher than that in a population of healthy children( Reference Shi, Berkemeyer and Buyken 30 ). Urinary free cortisol levels in the mothers in the present study were similar to those reported by Baudrand et al. ( Reference Baudrand, Campino and Carvajal 7 ), however, cortisol metabolite levels were lower. Differences between studies may reflect inter-laboratory differences in methodologies, which has been reported in the analysis of hair cortisol concentrations( Reference Stalder, Steudte-Schmiedgen and Alexander 31 ).
In the present study, our findings suggest for children that a high-Na diet is associated with an increase in daily production of cortisol. As previously reported, elevated cortisol levels have been linked to the development of chronic diseases in paediatric populations( Reference Heffelfinger and Newcomer 12 – Reference Papafotiou, Christaki and van den Akker 14 ). Our findings show that dietary Na appears to be a factor modifying cortisol production in both paediatric and adult populations. Our findings in mothers are consistent with evidence from studies in adults where high Na intake was also found to be positively associated with free urinary cortisol and urinary cortisol metabolites( Reference Baudrand, Campino and Carvajal 7 ). Likewise, in two studies in men that went from a low-Na (10 mmol/d) to high-Na diet (200 mmol/d), urinary cortisol increased by 65 nmol/d( Reference Litchfield, Hunt and Jeunemaitre 16 ) and 20 μg/d( Reference Chamarthi, Kolatkar and Hunt 18 ). Although there is evidence to suggest Na intake is linked to cortisol production, the precise mechanisms remain to be elucidated. The main pathways of cortisol metabolism include two isoforms of the 11β-hydroxysteroid dehydrogenase (11βHSD), 11βHSD1 and 11βHSD2, which are found in many human tissues( Reference Tomlinson and Stewart 9 ). 11βHSD1 converts inactive cortisone to cortisol and 11βHSD2 inactivates cortisol to cortisone. In rats fed a high salt diet, gene expression of 11βHSD1 increased in adipose tissue, resulting in greater production of the active hormone corticosterone (the main glucocorticoid found in rodents), providing support for how Na might influence cortisol secretion( Reference Usukura, Zhu and Yoneda 32 ). Currently, there is no evidence for an equivalent mechanism in a human model.
Our findings that urinary K was positively associated with urinary free cortisol and cortisol metabolites in children are novel as this has not been reported before in this population group. However, these findings were contrary to our hypothesis, where we predicted a negative association between urinary K and urinary free cortisol and total cortisol metabolites based on the reported health benefits of K. Nevertheless, feeding studies in rats have investigated a potential mechanism for how dietary K intake might influence corticosterone levels. Feeding rats a diet high in potassium chloride resulted in increased production of 11βHSD2 protein in the renal distal tubules, catalysing the conversion of the active corticosterone to the inactive 11-dehydrocorticosterone( Reference Hermans, Fischer and Schiffers 33 ). This results in greater specificity for renal distal tubule mineralocorticoid receptors to aldosterone, thereby promoting aldosterone-induced K excretion. In contrast, Thompson et al. ( Reference Thompson, Bailey and Michael 34 ) found that a high-K diet was associated with reduced renal 11βHSD2 activity in rats, thus, enhancing the access of glucocorticoids to the mineralocorticoid receptor to promote urinary K excretion. While these two studies show that K may be involved in 11βHSD2 regulation in the renal distal tubule, and therefore glucocorticoid excretion, the discordant findings suggest that further research is required in this area. Further studies are needed to determine the relevant mechanisms present in humans and any potential physiological or health implications.
It is important to consider the clinical relevance of our findings. We found that 1 mmol/24 h of urinary Na was related to a 0·31 nmol/24 h higher urinary free cortisol and that 1 mmol/24 h of urinary K was related to a 0·65 nmol/24 h higher urinary free cortisol. While reference ranges for cortisol are available for the diagnosis of clinical conditions such as Cushing’s syndrome( Reference Pappachan, Hariman and Edavalath 35 ), clinically meaningful levels for cortisol in terms of biological outcomes for non-clinical populations are not well understood. Studies in adults have found that decreasing Na by 180 mmol/24 h reduced urinary free cortisol by 40( Reference Lewicka, Nowicki and Vecsei 17 ) and 61 nmol/24 h( Reference Chamarthi, Kolatkar and Hunt 18 ). It is not likely, however, that such large reductions in Na would be achievable in the general population. In the present study, the average Na intake in the children was 104 mmol/d (compared with an upper level of 86 mmol/d, 9–13 years) and the average Na intake in the mothers was 121 mmol/d (compared with an upper level of 100 mmol/d)( 28 ). Overall, clinically meaningful reductions in urinary free cortisol are not well understood in paediatric or adult populations, therefore it is difficult to recommend a reduction in urinary Na and K; further research in this area is warranted.
In the present study, we found that urinary free cortisol and cortisol metabolites were positively associated between children and mothers. This is consistent with results from a study that found high maternal hair cortisol was strongly associated with their infant’s evening salivary cortisol levels( Reference Tarullo, St John and Meyer 36 ). In a sample of mothers and their children living in socioeconomically disadvantaged neighbourhoods, hair cortisol levels were also strongly positively associated between mothers and their children( Reference Olstad, Ball and Wright 37 ). Similarly, Pratt et al. ( Reference Pratt, Apter-Levi and Vakart 38 ) reported concordance for diurnal cortisol secretion between mothers and their 6-year-old children. Tarullo et al. ( Reference Tarullo, St John and Meyer 36 ) have proposed that this physiological concordance between mother and child has an evolutionary basis, such that having a similar response is adaptive when facing the same external risk.
There are limitations associated with this study, which must be considered when interpreting the findings. The cross-sectional design of the study prevents the detection of causal relationships between dietary electrolyte intake and cortisol levels. We did not obtain any anthropometric measures in the mothers, so we were unable to adjust for these in the regression analyses. While the association between Na or K and cortisol may be influenced by the types of foods and nutrients consumed by the children and mothers, we were unable to assess this in our study. The convenience sample of schoolchildren potentially limits the generalisability of the findings to a wider population. We only assessed mothers in this study as the relationship between urinary electrolytes and urinary cortisol and cortisol metabolites between mother and child may differ to father and child. A strength of this study was that both urinary cortisol and cortisol metabolites were measured to fully characterise cortisol production.
In conclusion, in a population of schoolchildren and their mothers, this study has shown that high Na and K intake is associated with increased production of cortisol. The negative health implications of elevated cortisol exposure are well documented. Future research should include metabolic studies to investigate mechanisms that might be involved and the effect of Na reduction on cortisol levels in these target populations.
Acknowledgements
The authors thank Dr Catherine Huggins for her early work in securing project funding. The authors acknowledge the Victorian Department of Early Childhood and Development for their support in allowing this study to be conducted within the government school sector. The authors thank all schools, children and mothers who participated in the study.
This study was funded by a National Heart Foundation of Australia Grant-in-Aid (G 10 M 5021), a Helen MacPherson Smith Trust Fund Project Grant (6002), a Deakin University Faculty of Health Research Development Grant and by seed funding from Deakin University’s Institute for Physical Activity and Nutrition. C. R. B. is supported by an Australian Research Council Future Fellowship (FT160100017).
C. G., C. A. N. and F. J. H. designed and conducted the original SONIC study. A. I. T., S. J. T. and C. A. N. designed and conducted this salt and cortisol sub-study including securing project funding. S. U. J. was responsible for the free urinary cortisol analysis. C. R. B. and S. A. M. were responsible for the urinary cortisol metabolite analysis. S. J. T. analysed the data and drafted the manuscript. All authors critically reviewed the manuscript and read and approved the final version.
None of the authors had a conflict of interest.










