Heart failure (HF) is the advanced manifestation of various CVD and portends poor prognosis, especially in patients who are hospitalised with worsening symptoms(Reference Zhang, Zhang and Butler1). In a survey reported in 2003, the prevalence of HF was 0·4, 0·9, 1·3 and 1·3 % among populations aged 35–44, 45–54, 55–64 and 65–74 years, respectively, reaching the estimation of more than 4 million HF patients aged 35–74 years in China(Reference HuShengshou and KongLingzhi2). HF is perhaps the most serious of all CVD and is a common cause of hospitalisation in China(Reference Jiang and Ge3, Reference Ariely, Evans and Mills4). It has a poor prognosis despite advances in treatment(Reference Jessup and Brozena5).
Vitamin D plays a role in skeletal health by regulating Ca and P metabolism. The active metabolite of vitamin D, 1α, 25-dihydroxyvitamin D, binds to the vitamin D receptor (VDR) that regulates numerous genes involved in fundamental processes of potential relevance to CVD(Reference Norman and Powell6). Vitamin D recently has been proposed to play an important role in cardiovascular health(Reference Anderson, May and Horne7). Vitamin D deficiency (VDD) has reached epidemic proportions; this deficiency has been observed to be highly prevalent in the HF community with rates varying from approximately 80 to 95 %(Reference Agarwal, Phan and Willix8).
There is growing evidence that suggests that vitamin D status is associated with the development and progression of HF(Reference Liu, Voors and van Veldhuisen9, Reference Dobnig, Pilz and Scharnagl10). Smaller clinical studies have suggested that low vitamin D status was prevalent in HF patients and is associated with poor outcome(Reference Boxer, Kenny and Cheruvu11, Reference Zittermann, Schleithoff and Gotting12). In patients with HF, low vitamin D levels were associated with adverse outcome and correlate with established clinical correlates and biomarkers(Reference Meems, Van der Harst and van Gilst13). A previous study(Reference Vacek, Vanga and Good14) found that VDD was a significant predictor of all-cause mortality and HF rehospitalisation in patients with mild to moderate HF. Another study found that VDD was a significant predictor of reduced survival(Reference Gotsman, Shauer and Zwas15).
25-Hydroxyvitamin D (25(OH)D) is usually considered as a circulating biomarker of vitamin D status. Melamed et al. (Reference Melamed, Michos and Post16) suggested that the lowest quartile of 25(OH)D level (<17·8 ng/ml) was independently associated with all-cause mortality in the general population, while another study found that serum 25(OH)D was an independent predictor of future adverse CHD events in patients with diabetes mellitus type 2(Reference Heidari, Nargesi and Hafezi-Nejad17). Furthermore, Liu et al. (Reference Liu, Chen and Hankins18) demonstrated that adults with inadequate serum 25(OH)D levels have significantly higher risk of death from HF and all CVD and all-cause premature death.
Interestingly, results evaluating 25(OH)D in ethnically homogeneous populations might not be broadly generalisable to other racial or ethnic groups(Reference Robinson-Cohen, Hoofnagle and Ix19). Data from Chinese sample studies about the prognostic value of serum 25(OH)D in patients with HF are rare, hence the purpose of the present study to assess the impact of serum concentrations of serum 25(OH)D on cardiac prognosis in Chinese patients with HF.
Patients and methods
Study population
Between 1 January 2015 and 31 December 2016, a series of patients with HF from the Heart Failure Clinic at Linyi People’s Hospital in China were recruited in this prospective study. The study was performed at geographic latitude 35·05° N. The diagnosis of HF was made based on typical signs and symptoms during hospitalisation followed by pulmonary congestion, pleural effusion or left ventricular (LV) enlargement by chest X-ray and/or echocardiography(Reference Funayama, Shishido and Netsu20). LV ejection fraction (LVEF) and LV end-diastolic diameter of all included patients were assessed through echocardiography. The included criteria were (1) aged over 45 years and (2) inpatients. Patients who used vitamin d/or Ca supplementation during the index year were excluded. There were no other exclusion criteria as long as the patients were willing to provide written informed consent.
For each patient, age, sex, BMI, blood pressure, the seasons into study, New York Heart Association (NYHA)-class, LVEF, LV end-diastolic diameter and medication status from the medical record along with information cause of HF were recorded.
End points and follow-up
Patients were followed prospectively for a median duration of 1 year. The endpoints were cardiac events, including CVD death and rehospitalisation for worsening HF. CVD death was identified by the Academic Research Consortium criteria(Reference Tu, Ma and Ni21), including death due to CVD such as CHD, congestive HF, sudden cardiac death or fatal stroke. Sudden cardiac death was defined as death without definite premonitory symptoms or signs and confirmed by the attending physician(Reference Narumi, Watanabe and Kadowaki22). A rehospitalisation was defined as an unplanned overnight stay in a hospital because of progression of HF or as a direct result of HF(Reference Liu, Voors and van Veldhuisen9). Cardiovascular events assessment was performed by two trained cardiologists. Blinded to the blood biomarker data, they reviewed the medical records and conducted telephone interviews with the patient or, if not possible, with the relative.
Measurement of serum concentrations of 25-hydroxyvitamin D and brain natriuretic peptide
The blood samples were prospectively drawn from the antecubital vein the first morning after admission. After centrifugation, the serum samples were immediately stored at –80°C before assay. Serum concentration of 25(OH)D was measured with competitive chemiluminescent immunoassay in a calibrated Elecsys 2010 (Roche Diagnostics GmbH), with intra- and inter-assay CV of 2·0–3·5 % and 2·5–4·0 %, respectively. The detection limit was 3 ng/ml. The 25(OH)D concentrations are therefore used to classify vitamin D status into VDD (< 20 ng/ml) and normal (≥ 20 ng/ml)(Reference Holick23). Serum brain natriuretic peptide (BNP) concentrations were measured using a commercially available ELISA method (Shiono RIA BNP assay kit, Shionogi & Co., Ltd). IL-6 was also tested by ELISA method. Other biochemical measurements were done using standard laboratory methods. Estimated glomerular filtration rate (eGFR) was calculated by an equation for the Chinese: eGFR (ml/min per 1·73 m2) = 175 × creatinine−1·234 ×age−0·179 × sex (male = 1, female = 0·19).
Sample size determination
A priori power analysis using data from previous research involving 25(OH)D(Reference Afarideh, Ghanbari and Noshad24) was conducted to determine the required number of participants (n). Subsequently, the study sample size was calculated using the equations from Whitley & Ball(Reference Whitley and Ball25). These calculations found that each group required eleven participants to meet the required power of 99 % at the α value ≤0·05. Therefore, the power of the study with more than forty-three participants for each group was calculated to be >99 % for a type 1 error of 0·05.
Data handling and statistical analysis
All data are expressed as medians and interquartile ranges (IQR) or numbers and percentages. P < 0·05 was considered statistically significant (two sided). Statistical analysis was performed with SPSS for Windows, version 22.0 (SPSS Inc.) and the ROCR package (version 1.0-2).
The χ2 and Mann–Whitney U test were applied for comparing the proportions and medians values between groups. Correlations between 25(OH)D and other parameters were studied by Spearman correlation analysis. Univariate and multivariate analyses with Cox proportional hazard regression was used for assessing the relationship between serum concentration of 25(OH)D and cardiac events and CVD mortality. The results were revealed as hazard ratios (HR) with 95 % CI. For a more detailed exploration of the 25(OH)D and outcome, multivariate analysis models to estimate adjusted HR and 95 % CI of cardiac events or CVD mortality for 25(OH)D quartiles (the 4th quartile as reference) were applied. Furthermore, the relationship between VDD and cardiac events or CVD mortality had also been presented.
Moreover, the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) were used to measure the quantity of improvement for the correct classification according to the addition of serum 25(OH)D concentrations to the prediction model. Last, cumulative cardiac events and survival rates were computed using the Kaplan–Meier method and were compared using the log-rank test. We divided the patients into four groups according to the 25(OH)D quartiles.
Ethics
The design of the present study was reviewed and approved by the investigational review board of the Linyi People’s Hospital. Informed consents were obtained from patients prior to their inclusion in the present study according to the guidance of Declaration of Helsinki.
Results
Baseline clinical characteristics
In the present study, 343 patients were included and finished the follow-up. The 25(OH)D concentration was obtained from the patients with a median value of 17·4 (IQR 12·6–23·4) ng/ml. A total of 221 patients were defined as VDD; thus, the prevalence was 64·4 (95 % CI 59·4, 69·5) %. The data suggested significant seasonal differences in 25(OH)D concentrations, and 25(OH)D concentrations were lower in the winter than in other seasons (15·4 (IQR 10·4–19·9) v. 18·6 (IQR 13·2–24·7); P = 0·021). The median value of LVEF was 46 (IQR 34–55) %, while the LV end-diastolic diameter was 58 (IQR 47–69) mm. In addition, the overall use of β blockers, diuretics, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, statins and Ca channel blockers at admission was 67·9, 89·6, 74·3, 35·6 and 21·0 %, respectively. Baseline clinical characteristics are presented in Table 1.
Serum 25-hydroxyvitamin D concentrations and heart failure severity, other biomarkers
The patients who were in NYHA functional class IV found significantly lower serum concentrations of 25(OH)D compared with those in classes II and III (P = 0·001 v. class III and P < 0·001 v. class II, Fig. 1). As a continuous variable, a correlation between NYHA score and serum concentrations of 25(OH) was found (r (Spearman) −0·303; P < 0·001). Negative associations between 25(OH)D and age (r −0·211, P = 0·015), IL-6 (r −0·334, P < 0·001), LV end-diastolic diameter (r −0·269, P = 0·009), CRP (r −0·311, P < 0·001), glucose (r −0·233, P = 0·012) and BNP (r −0·338, P < 0·001) were also obtained. Furthermore, positive associations between 25(OH)D and LVEF (r 0·355, P < 0·001) and eGFR (r 0·198, P = 0·032) were recorded.
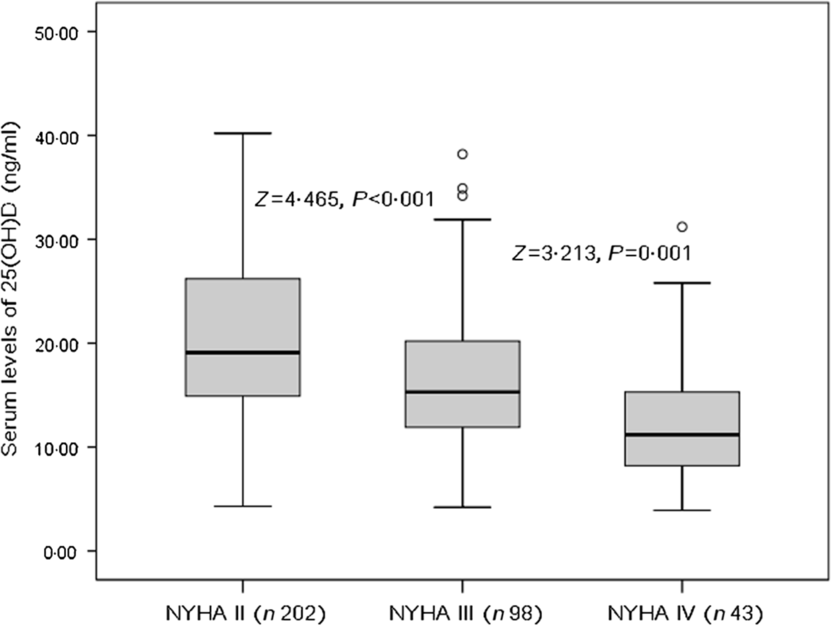
Fig. 1. Serum 25-hydroxyvitamin D (25(OH)D) concentrations and heart failure severity (defined by New York Heart Association (NYHA) functional class).
Comparison between heart failure patients with and without cardiac events
There were 102 cardiac events, including forty-three deaths and fifty-nine rehospitalisations in patients with HF during the 1-year follow-up period. As shown in Table 2, patients who experienced cardiac events were older, in a more severe NYHA functional class and had a lower BMI, lower eGFR, lower LVEF, higher LV end-diastolic diameter and higher serum concentrations of glucose, CRP, IL-6 and BNP compared with those who did not. In addition, cardiac events were experienced more likely during winter. Moreover, Fig. 2(a) reveals that patients with cardiac events had lower serum concentrations of 25(OH)D compared with those without (13·2 (IQR 9·0–16·4) v. 19·1 (IQR 14·9–25·8) ng/ml).
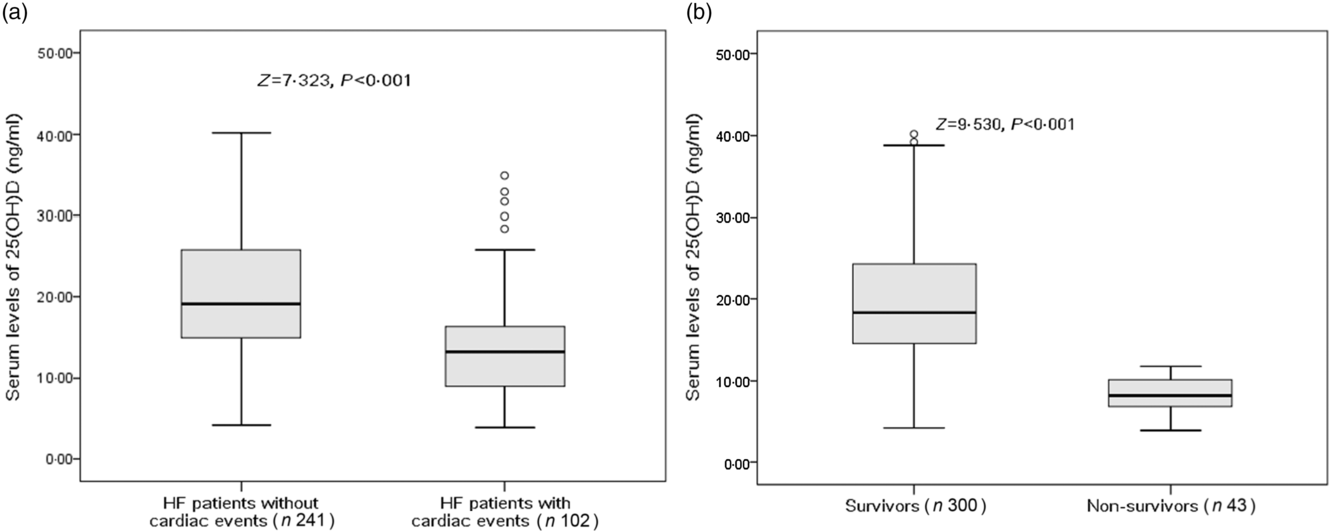
Fig. 2. Comparisons of serum 25-hydroxyvitamin D (25(OH)D) concentrations between different groups. (a) Comparisons of serum 25(OH)D concentrations between heart failure (HF) patients with and without cardiac events; (b) comparisons of serum 25(OH)D concentrations between survivors and non-survivors of HF. Data were analysed by the Mann–Whitney U test. All data are medians and interquartile ranges.
Table 1. Baseline clinical characteristics (Numbers of patients; medians and interquartile ranges (IQR); numbers and percentages)
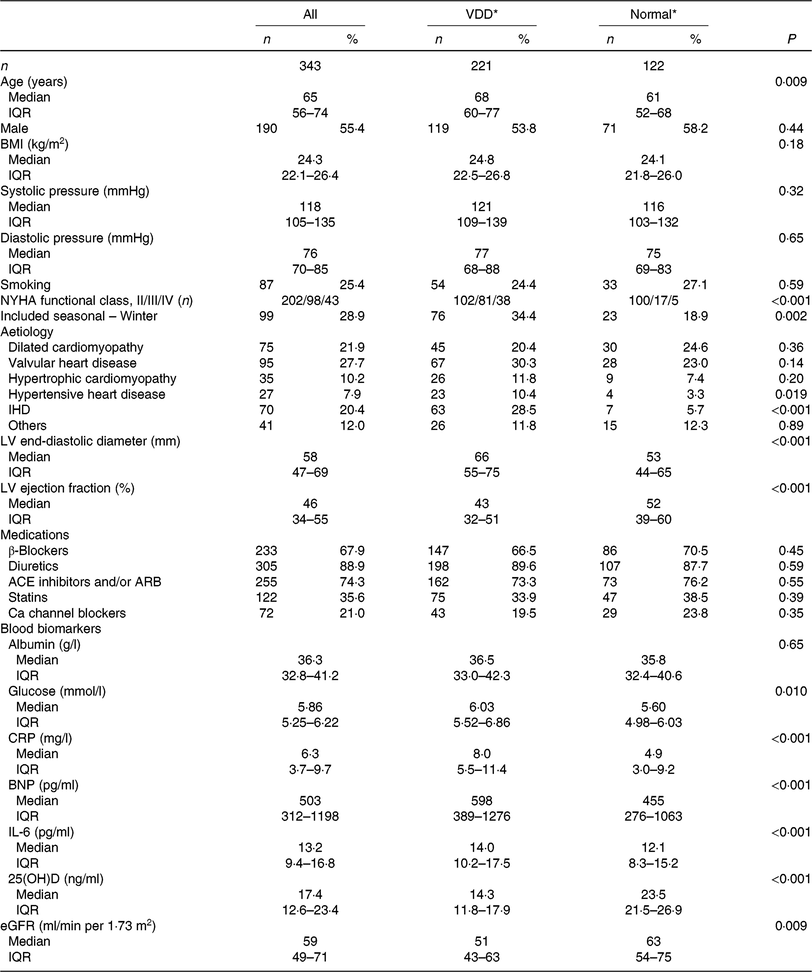
VDD, vitamin D deficiency; NYHA, New York Heart Association; LV, left ventricular; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CRP, C-reactive protein; BNP, brain natriuretic peptide; 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration rate.
* The 25(OH)D concentrations are used to classify vitamin D status into VDD (< 20 ng/ml) and normal (≥ 20 ng/ml).
Table 2. Univariate analyses for cardiac event and mortality (Hazard ratios (HR) and 95 % confidence intervals)
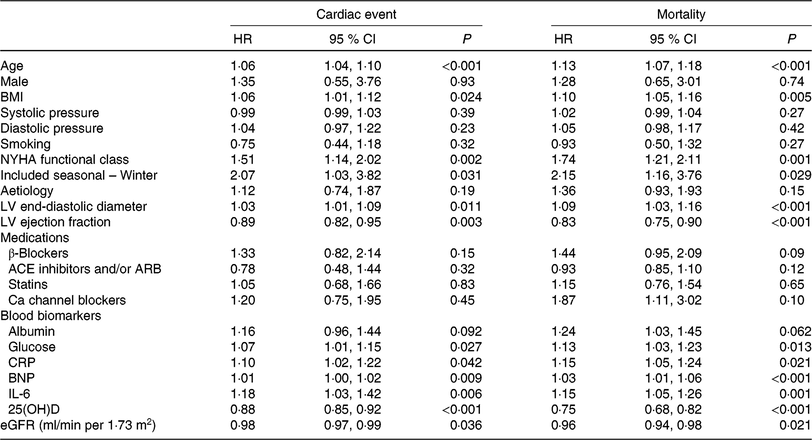
NYHA, New York Heart Association; LV, left ventricular; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CRP, C-reactive protein; BNP, brain natriuretic peptide; 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration rate.
Association between serum concentrations of 25-hydroxyvitamin D and cardiac events
As a continuous variable, 25(OH)D was associated with decreased risk of cardiac events (OR 0·88, 95 % CI 0·85, 0·92; P < 0·001) in the univariate model. In multivariable Cox regression analysis, 25(OH)D was still associated with decreased risk of cardiac events (HR 0·93, 95 % CI 0·88, 0·97; P = 0·003) after adjusting for other significant factors, confirmed in Table 2, including age, BMI, NYHA functional class, seasonal variation, LV end-diastolic diameter, LVEF, eGFR, serum concentration of glucose, C-reactive protein (CRP), BNP and IL-6. The cardiac events increased significantly across decreasing 25(OH)D quartiles from 9·3 to 55·8 % (P < 0·001; Table 3). In addition, multivariate analysis models were used to assess cardiac events according to 25(OH)D quartiles (the highest quartile (Q4) as the reference), with the adjusted HR with 95 % CI were reported. As shown in Table 3, the 1st and 2nd quartiles of 25(OH)D were compared against Q4 and the HR were 6·68 (95 % CI 3·32, 11·16; P < 0·001) and 3·36 (95 % CI 1·98, 7·16; P = 0·001), respectively. Furthermore, the association between VDD status and cardiac events had been presented. Interestingly, VDD was a predictor of cardiac events, with the unadjusted and adjusted risks increased by 363 % (HR 4·63; 95 % CI 2·53, 8·47; P < 0·001) and 203 % (HR 3·03; 95 % CI 1·59, 5·19; P = 0·003), respectively.
Table 3. Univariate and multivariate analyses for cardiac events (CE) and mortality according to 25-hydroxyvitamin D (25(OH)D) quartiles* (Numbers and percentages; hazard ratios (HR) and 95 % confidence intervals)
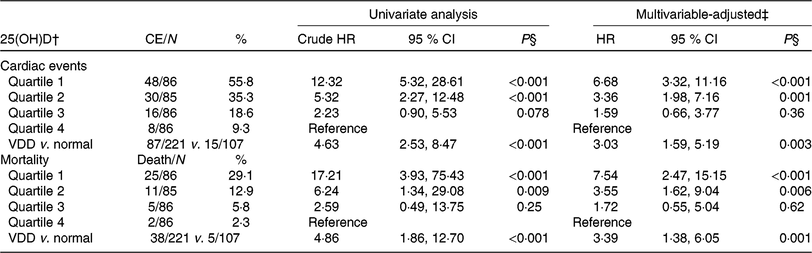
VDD, vitamin D deficiency; HF, heart failure; NYHA, New York Heart Association; LV, left ventricular; LVEF, LV ejection fraction; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; BNP, brain natriuretic peptide.
* Cardiac events included CVD deaths and rehospitalisations for worsening HF.
† 25(OH)D in quartile 1 (< 12·6 ng/ml), quartile 2 (12·6–17·4 ng/ml), quartile 3 (17·5–23·4 ng/ml) and quartile 4 (>23·4 ng/ml). VDD was defined as 25(OH)D < 20·0 ng/ml.
‡ Adjusted for significant factors confirmed in Table 2, including age, BMI, NYHA functional class, included seasonal, LV end-diastolic diameter, LVEF, eGFR, serum concentration of glucose, CRP, BNP and IL-6.
§ P value for trend < 0·001.
The NRI and the IDI were used to test the quantity of improvement for the correct reclassification according to the addition of 25(OH)D serum concentrations to the prediction model I (includes age, BMI, NYHA functional class, seasonal variation, LV end-diastolic diameter, LVEF, eGFR, serum concentration of glucose, CRP, BNP and IL-6). Thirty-two patients with cardiac events were classified in higher risk categories using the inclusion of 25(OH)D in the prediction model I. Fourteen patients with cardiac events were classified in lower risk categories using the model with the 25(OH)D and model I as compared with the model with the model I as the only predictor variable. As shown in Table 4, the inclusion of 25(OH)D in the prediction model I for the prediction of cardiac events enhanced the NRI (P = 0·003) and IDI (P < 0·001) values, confirming the effective reclassification and discrimination.
Table 4. Statistics for model fit and improvement with addition of vitamin D deficiency (VDD) predicted on the prediction of cardiac events and mortality*
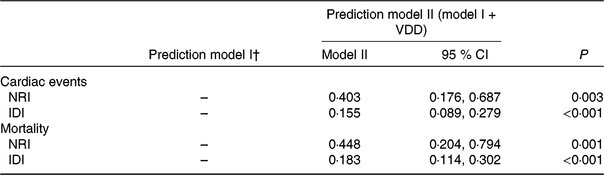
NRI, net reclassification improvement; IDI, integrated discrimination improvement; 25(OH)D, 25-hydroxyvitamin D; NYHA, New York Heart Association; LV, left ventricular; LVEF, LV ejection fraction; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; BNP, brain natriuretic peptide.
* VDD was defined as 25(OH)D < 20·0 ng/ml.
† Prediction model I included age, BMI, NYHA functional class, included seasonal, LV end-diastolic diameter, LVEF, eGFR, serum concentration of glucose, CRP, BNP and IL-6.
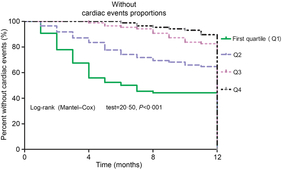
Furthermore, as shown in Fig. 3, the patients were divided into four groups according to the 25(OH)D quartiles. Kaplan–Meier analysis suggested that patients with a lower serum 25(OH)D concentrations had a higher risk of cardiac events (log-rank test P < 0·001).

Fig. 3. Kaplan–Meier analysis of cardiac events according to the 25-hydroxyvitamin D (25(OH)D) quartiles. The patients with lower serum 25(OH)D concentrations had a higher risk of cardiac events (log-rank test P < 0·001).
Subgroup analysis
Among the 102 cardiac events, forty-three of them died. The serum concentration of 25(OH)D in the cases of death was only half when compared with those cases of survival (10·7 (IQR 7·2–14·8) v. 18·2 (IQR 13·8–24·1) ng/ml; P < 0·001). As a continuous variable, 25(OH)D was associated with mortality; when 25(OH)D was increased by each 1 ng/ml, the unadjusted and adjusted risk were decreased by 25 % (HR 0·75; 95 % CI 0·68, 0·82) and 17 % (HR 0·83; 95 % CI 0·77, 0·89), respectively. Similarly, the association between VDD status and mortality also had been proposed. VDD was a predictor of mortality and the unadjusted and adjusted risks were increased by 386 % (HR 4·86; 95 % CI 1·86, 12·70; P < 0·001) and 239 % (HR 3·39; 95 % CI 1·38, 6·05; P = 0·001), respectively (Table 3).
Again, the NRI and the IDI were used to test the quantity of improvement for the correct reclassification according to the addition of 25(OH)D serum concentrations to the prediction model I. Nine patients who died were classified in higher risk categories and two patients who died were classified in lower risk categories using the model with the model I and 25(OH)D level as compared with the model with the model I as the only predictor variable. As shown in Table 4, the inclusion of 25(OH)D in the prediction model I for the prediction of mortality enhanced the NRI (P = 0·001) and IDI (P < 0·001) values, confirming the effective reclassification and discrimination. Furthermore, as shown in Fig. 4, the patients were divided into four groups according to the 25(OH)D quartiles. Kaplan–Meier analysis suggested that the patients with a lower serum 25(OH)D concentrations had a higher risk of mortality (log-rank test P = 0·032).
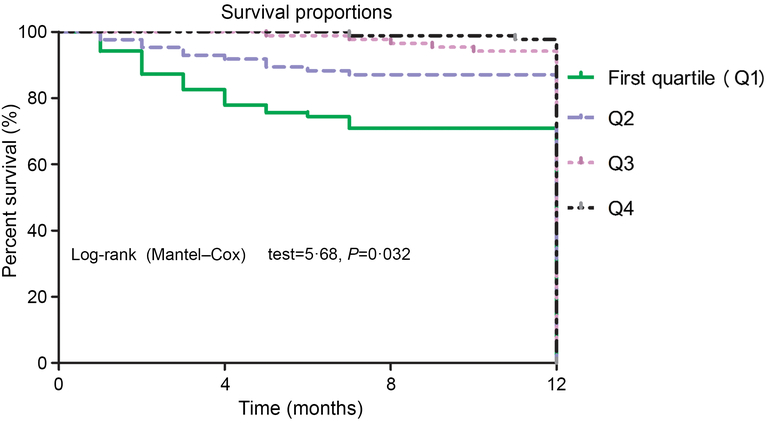
Fig. 4. Kaplan–Meier analysis of CVD mortality according to the 25-hydroxyvitamin D (25(OH)D) quartiles. The patients with lower serum 25(OH)D concentrations had a higher risk of CVD mortality (log-rank test P = 0·032).
During the follow through, eight patients experienced non-cardiovascular deaths (six patients died from cancer and two from other causes). Adjusted analyses were repeated for all-cause mortality and similar results were obtained with a HR for VDD of 4·24 (95 % CI 1·69, 8·03; P < 0·001). Similarly, the inclusion of 25(OH)D in the prediction model I for the prediction of all-cause mortality enhanced the NRI (P = 0·009) and IDI (P = 0·002) values, confirming the effective reclassification and discrimination.
Discussion
The steroid hormone vitamin D regulates gene expression of many genes that play a prominent role in the progression of HF such as cytokines and hormones(Reference Meems, Van der Harst and van Gilst13). In the present study, we reported the prevalence of VDD in a Chinese cohort of HF patients and described the cardiac prognostic value in this population. The present study found that serum concentration of 25(OH)D was a novel prognostic marker for the risk stratification of patients with HF. More importantly, VDD was a significant predictor of increased cardiac events and reduced survival in patients with HF.
Consistent with our finding, Schierbeck et al. (Reference Schierbeck, Jensen and Bang26) highlighted that vitamin D was independently associated with all-cause and CVD mortality in patients with HF in a relatively small prospective study (n 148), while another study confirmed that a low 25(OH)D concentration was associated with a poor prognosis in HF patients(Reference Liu, Voors and van Veldhuisen9). Similar findings were found in a study of 220 ischaemic stroke patients where higher. The 90-d mortality rates were significantly associated with lower levels of 25(OH)D(Reference Tu, Zhao and Xu27). Another study demonstrated that VDD was associated with increased arterial wall stiffness in a young population, suggesting the increased risk for all-cause mortality in vitamin D deficient subjects(Reference Dong, Stallmann-Jorgensen and Pollock28). Kestenbaum et al. (Reference Kestenbaum, Katz and De Boer29) found that serum 25(OH)D concentrations < 15 ng/ml were associated with a 29 % greater risk for mortality among older adults. In a meta-analysis of 77 155 participants of mixed ethnicity, the risk of any early death was increased by 35 (95 % CI 31, 42) % for the lowest v. the highest quartile of 25(OH)D level(Reference Brøndum-Jacobsen, Benn and Jensen30). A population-based study of 9146 younger adults found significant associations with all-cause mortality but not CVD mortality(Reference Skaaby, Husemoen and Pisinger31). In contrast, no association between vitamin D and mortality was found in a prospective study of older men(Reference Cawthon, Parimi and Barrett-Connor32). Similarly, Gerling et al. (Reference Gerling, James and Wilton33) found that serum total 25-OH vitamin D was inversely associated with mortality risk, and this association was attenuated by several competing risk factors. They suggested that overall 25-OH vitamin D added little prognostic value over established risk factors. In the present study, we calculated the NRI and found that, even though 25(OH)D was associated with mortality, it added very little additional prognostic value.
The prevalence of VDD (25(OH)D levels < 20 ng/ml) was present in 75·2 % of the 10 100 people aged 40–75 years in Lanzhou, China(Reference Zhen, Liu and Guan34). Overall, in the present study, 64·4 % of the HF was defined as VDD (25(OH)D levels < 20 ng/ml) and 86·6 % had 25(OH)D levels < 30 ng/ml. Similarly, another study found that 75 % of patients had VDD by most guidelines (<20 ng/ml)(Reference Liu, Voors and van Veldhuisen9). Among subjects with chronic HF, 97·8 % presented VDD (25(OH)D < 30 ng/ml) and severe (<10 ng/ml) in 66·7 %(Reference Ameri, Ronco and Casu35). However, another study reported that VDD (≤20 ng/ml) was prevalent in 43 % of the 148 HF outpatients(Reference Schierbeck, Jensen and Bang26). There were variations in sites of study, sampling and testing methods, including criteria, racial or ethnic, culture, dietary and economic status between the various studies. Those variations make it difficult to compare different studies. In general, VDD is a common presence in patients with HF.
The association between low vitamin D and an activated RAS and inflammatory status had been suggested(Reference Liu, Voors and van Veldhuisen9). In the present study, we also found an inverse correlation between 25(OH)D and inflammatory status (CRP and IL-6). Two previous studies had found that high levels of CRP were a common finding in patients with HF and were associated with a poor prognosis(Reference Cesari, Penninx and Newman36, Reference Boxer, Dauser and Walsh37), which was supported by our finding. In addition, a study revealed a sex-specific association between VDD and LV dilation(Reference Ameri, Ronco and Casu35), in which our research had similar findings.
The relationship between decreasing low concentrations of 25(OH)D and cardiac prognosis in patients with HF reported in the present study was no proof of a causal relation due to the observational design; however, there are mechanisms through which low concentrations of 25(OH)D could have harmful effects on the cardiovascular system. First, vitamin D was converted into an active metabolite calcitriol (1,25-hydroxyvitamin D3) in the kidney. Calcitriol is a negative regulator of the renin–angiotensin system and cardiomyocyte proliferation, and suppresses immune and inflammatory responses(Reference Gotsman, Shauer and Zwas15). Calcitriol is also directly involved in Ca-dependent cellular processes, including the synthesis of Ca-binding protein, the activation of adenylate cyclase, the rapid activation of voltage-dependent Ca channels and the influx, reuptake and release of Ca from the sarcoplasmic reticulum(Reference Selles, Bellido and Boland38). This altered intracellular handling of ionised Ca could also contribute to the impaired contractility of the myocardium in HF patients(Reference Beuckelmann, Nabauer and Erdmann39). VDR knockout mice develop typical signs of HF, including activation of the renin–angiotensin–aldosterone system, cardiac hypertrophy, high blood pressure and increased levels of atrial natriuretic peptide(Reference Xiang, Kong and Chen40). Second, 25(OH)D controls inflammatory and immune responses keeping them within physiological boundaries(Reference Norman and Powell6). An important feature of the modulation of immune function by 25(OH)D is suppression of the development and responses of TH1 and TH17 cells and favouring Treg and TH2 cells(Reference Takeda, Yamashita and Sasaki41). Proinflammatory cytokine are down-regulated and the anti-inflammatory ones such as IL-4 and IL-10 are up-regulated(Reference Dobnig, Pilz and Scharnagl10, Reference Cesari, Penninx and Newman36–Reference Boxer, Dauser and Walsh37). Third, a relationship between vitamin D status and endothelial function had been suggested. Vitamin D may attenuate the adverse effects (including increased NFκB expression) of advanced glycation end-products on endothelial cells(Reference Tarcin, Yavuz and Ozben42). A previous study demonstrated that 25(OH)D down-regulated tissue factor and up-regulated thrombomodulin expression in cultured monocytic cells via a VDR-mediated mechanism(Reference Gerling, James and Wilton33). Last, parathyroid hormone is elevated in patients with VDD. Parathyroid hormone has been shown to be an independent predictor of all-cause and cardiovascular mortality in patients with HF(Reference Schierbeck, Jensen and Bang26). In addition, there was experimental evidence that VDD results in maladaptive cardiac remodelling attributable to progressive myocyte hypertrophy and interstitial fibrosis(Reference Norman and Powell6).
A more meaningful study was whether vitamin supplementation can improve the prognosis, especially for HF patients with VDD. However, our cross-sectional design could not obtain any causal relation. It remains unclear if vitamin D supplementation is beneficial in preventing HF or if it could be a therapeutic addendum in the treatment of HF(Reference Meems, Van der Harst and van Gilst13). Unfortunately, vitamin D supplementation trials show mixed results. Interestingly, a previous study suggested that vitamin D supplementation has great benefits as an anti-inflammatory agent in infants with chronic HF by helping acceleration of the clinical improvement and cytokine profile balance(Reference Shedeed43). Previous studies found that vitamin D supplementation was significantly associated with better survival, specifically in patients with documented deficiency(Reference Vacek, Vanga and Good14), and vitamin D may be an important adjunct to standard HF therapy(Reference Boxer, Hoit and Schmotzer44) and improve quality of life (QOL)(Reference Moretti, Colucci and Berry45). In contrast, a study found that treatment with Vitamin D supplementation did not show clear evidence of benefit for preventing HF or influencing its clinical course(Reference D’Amore, Marsico and Parente46), while another study found that vitamin D supplementation has no beneficial effects on improvement of LV function and exercise tolerance in chronic HF patients(Reference Jiang, Gu and Zhang47). Witham et al. (Reference Witham, Crighton and Gillespie48) found that vitamin D supplementation did not improve functional capacity or QOL in a short-term trial of 105 older patients with HF and vitamin D insufficiency. These results bring into questions whether vitamin D is a risk factor for HF, a marker of HF disease severity or has a true pathological role. The influence of vitamin D on all these pathways in models of HF merits further exploration.
A limitation of the present study was the relatively small sample size, which made it impossible to draw firm conclusions with regard to hard endpoints. In addition, we only studied a Chinese HF population. Second was that the present study analysed a single measurement of 25(OH)D, which may not adequately reflect the long-term vitamin D status in these patients. In addition, newer studies suggest that 30 ng/ml was a better indicator of sufficiency; this has been proposed as the minimum level needed to suppress parathormone (PTH) maximally(Reference Schierbeck, Jensen and Bang26). In the present study, 20 ng/ml was used. Third, as there is a strong link between renal function and heart disease, and since the kidneys transform vitamin D to its active form, it is likely that there will be an interaction between the prognostic importance of vitamin D, PTH and renal function(Reference Cozzolino, Ketteler and Zehnder49). Many studies reported that PTH was the truly important role as a prognostic factor in HF. We were not able to identify this interaction, most likely due to fact that the PTH concentration was not assessed. Fourth, some severe deterioration of LVEF might have been excluded due to the use of vitamin and/or Ca supplementation. In addition, we might include mostly the patients with HF preserved, not reduced, ejection fraction. Judging the HF occurrence only on the basis of clinical symptoms may be sometimes misleading. Last, any causal relationship could not be proved due to the observational study design. We could not determine that VDD was a marker of worse outcomes or a biomarker of inadequately treated. The true mechanism of VDD might be that the patients with raised NYHA class were more prone to be immobilised; thus, the synthesis of active forms of vitamin D was impaired. The addition of the simple survey of declared sun exposure could have positive impact on the methodology of the study. Whether maintaining the serum concentration of vitamin D to normal would be beneficial for prognosis requires further controlled clinical trials to confirm.
Conclusion
In short, decreased serum concentrations of 25(OH)D were associated with cardiac prognosis and CVD mortality in a Chinese population with HF independent of other baseline HF markers. Serum 25(OH)D concentration appears to represent a novel prognostic marker for the risk stratification of patients with HF. Since it plays an important role in the multiple stages of HF progression, 25(OH)D is suggested as a prognostic biomarker and worthy of further research as a possible therapeutic target.
Acknowledgements
We are grateful to the staff in the Department of Cardiology, the Linyi People’s Hospital, for their support with patient recruitment. We are also grateful to the patients who were included the present study. The authors also acknowledge the contribution of the reviewers who have helped us improve the manuscript.
L. H.-Y. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: H. Y.-M., Z. J.-Y., L. H.-Y.; acquisition of data: H. Y.-M., L. H.-Y.; analysis and interpretation of data: H. Y.-M., Z. J.-Y.; drafting of the manuscript: H. Y.-M.; critical revision of the manuscript for important intellectual content: Z. J.-Y., L. H.-Y.; administrative, technical or material support: H. Y.-M., Z. J.-Y., L. H.-Y.; study supervision: H. Y.-M.
There were no conflicts of interest.