Article contents
Molecular and catalytic properties of an arginine kinase from the nematode Ascaris suum
Published online by Cambridge University Press: 25 July 2011
Abstract
We amplified the cDNA coding for arginine kinase (AK) from the parasitic nematode Ascaris suum, cloned it in pMAL plasmid and expressed the enzyme as a fusion protein with the maltose-binding protein. The whole cDNA was 1260 bp, encoding 400 amino acids, and the recombinant protein had a molecular mass of 45,341 Da. Ascaris suum recombinant AK showed significant activity and strong affinity 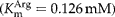 for the substrate l-arginine. It also exhibited high catalytic efficiency
for the substrate l-arginine. It also exhibited high catalytic efficiency 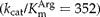 comparable with AKs from other organisms. Sequence analysis revealed high amino acid sequence identity between A. suum AK and other nematode AKs, all of which cluster in a phylogenetic tree. However, comparison of gene structures showed that A. suum AK gene intron/exon organization is quite distinct from that of other nematode AKs. Phosphagen kinases (PKs) from certain parasites have been shown to be potential novel drug targets or tools for detection of infection. The characterization of A. suum AK will be useful in the development of strategies for control not only of A. suum but also of related species infecting humans.
comparable with AKs from other organisms. Sequence analysis revealed high amino acid sequence identity between A. suum AK and other nematode AKs, all of which cluster in a phylogenetic tree. However, comparison of gene structures showed that A. suum AK gene intron/exon organization is quite distinct from that of other nematode AKs. Phosphagen kinases (PKs) from certain parasites have been shown to be potential novel drug targets or tools for detection of infection. The characterization of A. suum AK will be useful in the development of strategies for control not only of A. suum but also of related species infecting humans.
- Type
- Research Papers
- Information
- Copyright
- Copyright © Cambridge University Press 2011
References
- 7
- Cited by




