Article contents
Thermal expansion of nano–boron carbide under constant DC electric field: An in situ energy dispersive X-ray diffraction study using a synchrotron probe
Published online by Cambridge University Press: 03 January 2020
Abstract
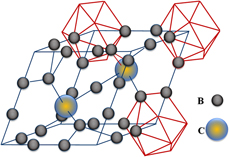
The thermal expansion coefficient (TEC) of nano-B4C having 50 nm mean particle size was measured as a function of applied direct current (DC) electric field strength varying from 0 to 12.7 V/mm and over a temperature range from 298 K up to 1273 K. The TEC exhibits a linear variation with temperature despite being measured over a range that is well below 50% of B4C’s normal melting temperature. The zeroth- and first-order TEC coefficients under zero-field condition are 4.8220 ± 0.009 × 10−6 K−1 and 1.462 ± 0.004 × 10−9 K−1, respectively. Both TECs exhibit applied DC electric field dependence. The higher the applied field strength, the steeper the linear thermal expansion response in nano-B4C, which suggests that the applied field affects the curvature of the interatomic potentials at the equilibrium bond length at a given temperature. No anisotropic thermal expansion with and without applied electric field was observed, although nano-B4C has a rhombohedral unit cell symmetry. The rhombohedral unit cell angle was determined as δR= 65.7046° (0.0007), and it remains unaffected by a change in temperature and applied electric field strength, which we attribute to B4C nanoparticle size and its carbon saturation.
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 1: Focus Section: Advances in Battery Technology: Material Innovations in Design and Fabrication , 14 January 2020 , pp. 90 - 97
- Copyright
- Copyright © Materials Research Society 2020
References
- 12
- Cited by




