To the Editor—Clostridioides difficile infection (CDI) represents a significant healthcare challenge, with 10%–30% of patients developing at least 1 recurrent episode, and the risk of relapses increases with each subsequent recurrence.Reference McDonald, Gerding and Johnson 1 Recurrent CDI is defined by resolution of CDI symptoms while on treatment, followed by symptom reappearance within 2–8 weeks after treatment completion.Reference McDonald, Gerding and Johnson 1 Exposure to systemic antibiotics in patients with prior CDI has been identified as an independent risk factor associated with CDI recurrence.Reference McDonald, Gerding and Johnson 1 Despite the lack of robust supporting evidence, secondary prophylaxis with oral vancomycin in patients on systemic antibiotics for infections other than CDI has become common practice.Reference Van Hise, Bryant, Hennessey, Crannage, Khoury and Manian 2 – Reference Caroff, Menchaca and Zhang 4 We read “Oral vancomycin prophylaxis (OVP) during systemic antibiotic exposure to prevent Clostridioides difficile infection relapses” by Caroff et alReference Caroff, Menchaca and Zhang 4 with great interest. The authors reported that OVP was effective in reducing CDI relapse in patients with incident CDI (1 previous CDI) but not recurrent CDI (≥2 CDI episodes).
We applaud the authors for conducting this study and taking into account potential confounders that were not adequately addressed in previous observational studies.Reference Van Hise, Bryant, Hennessey, Crannage, Khoury and Manian 2 , Reference Carignan, Poulin and Martin 3 In a small single-center study (n = 203), Van Hise et alReference Van Hise, Bryant, Hennessey, Crannage, Khoury and Manian 2 reported that OVP decreased the risk of CDI relapse (odds ratio, 0.12; 95% CI, 0.04–0.4), though they did not differentiate between incident and recurrent CDI with multivariate analysis. Carignan et alReference Carignan, Poulin and Martin 3 demonstrated that the benefit of OVP in preventing CDI relapse was confined to patients with recurrent CDI. Importantly, the significant differences between the Carignan and Caroff investigations make it difficult to draw definitive conclusions, even in these larger-scale multicenter studies (Table 1).
Table 1. Comparison of Oral Vancomycin Prophylaxis (OVP) During Systemic Antibiotic Exposure in Preventing CDI Relapses
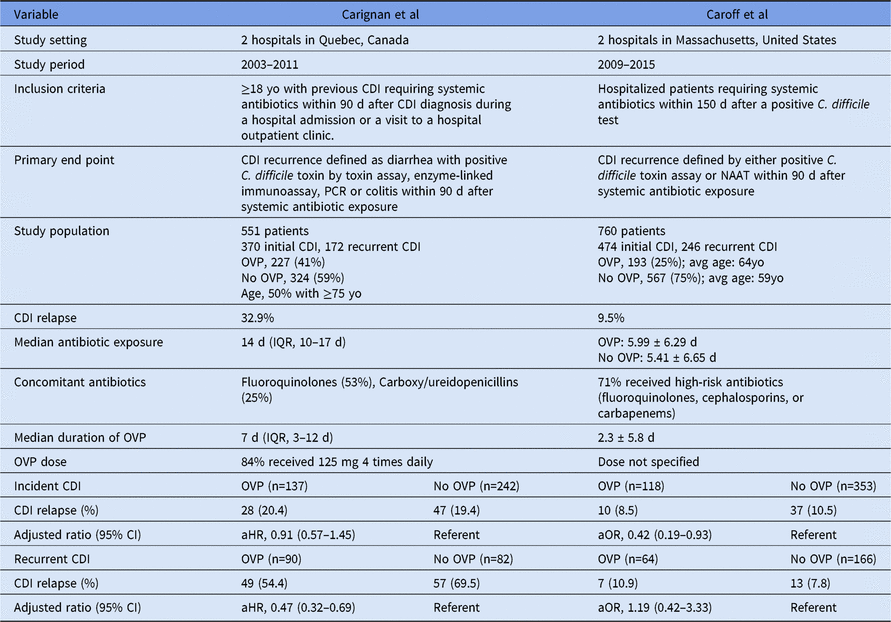
Note. aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CDI, Clostridium difficile infection; CI, confidence intervals; IQR, interquartile range; NAAT, nucleic acid amplification test; OVP, oral vancomycin prophylaxis; PCR, polymerase chain reaction.
In the Caroff study, the inclusion of hospitalized patients within 150 days of a positive C. difficile test did not capture ambulatory CDI cases, which may have explained the reported relapse rate of 9.5%, which is substantially lower than the historical epidemiology of CDI.Reference McDonald, Gerding and Johnson 1 This low recurrence rate also raises questions about the circulating C. difficile strain type. NAP1 (North American pulsed-field gel electrophoresis type-1) strains are often a predictor of disease severity, recurrence, and increased mortality.Reference McDonald, Gerding and Johnson 1 The CDI relapse rate of 32.9% reported by Carignan et al may have overlapped with the NAP1 outbreak in Quebec, Canada, in 2002Reference McDonald, Gerding and Johnson 1 and potentially with NAP1 strains still circulating in the community during their study periods between 2003 to 2011. Because PCR ribotyping was not available in the OVP studies, it is unclear whether NAP1 strain may have contributed to the observed differences in relapse rates. The study populations are somewhat different as well: 50% of the patients were >75 years old in the Carignan study, whereas the average age in the Caroff study was 59–64 years old. In addition to older age being a risk factor for CDI recurrence, older adults may have impaired host defense because neutrophils are less able to phagocytize and kill C. difficile.Reference Carignan, Poulin and Martin 3 This difference may partially indicate that host factors and humoral immunity play a role in CDI relapses.
Risk stratification of antibiotic class and duration of systemic antibiotic therapy should also be considered when calculating the risk of CDI recurrence given evidence suggesting that cumulative antibiotic exposures (ie, antibiotic dose, number of antibiotics, and days of antibiotic exposure) all appear to be associated with CDI risk.Reference Stevens, Dumyati, Fine, Fisher and van Wijngaarden 5 Caroff et al classified antibiotic risk as high, medium, and low, but the median duration of systemic antibiotic exposure was substantially shorter (6 days vs 14 days) than in the Carignan study. This difference led us to question whether a minimum threshold of antibiotic exposures is needed to derive benefit from OVP.
Because oral vancomycin may have deleterious effects to the indigenous microbiota of the colon and may promote colonization with vancomycin resistant Enterococci (VRE), carbapenem-resistant Klebsiella pneumoniae (KPC), and E. coli, it is imperative to carefully weigh the risks and benefits to determine the optimal dose and duration of OVP.Reference Lewis, Buffie and Carter 6 The duration of OVP was much shorter (2.3 days vs 7 days) in the Caroff study, and vancomycin dose was not specified. The optimal dose of OVP has yet to be defined, and various dosing regimens for CDI may be used, for example 125 mg 4 times daily in the Carignan’s study and 125–250 mg twice daily in the Van Hise study. With the increasing recognition of vancomycin’s effect on the host microbiome, it seems prudent to define the lowest effective dose of OVP to minimize collateral damage. Because oral vancomycin is not systemically absorbed, high colonic vancomycin levels (500–1000 µg/mL) can be achieved with 125 mg 4 times daily, which is several hundred-fold higher than the vancomycin minimum inhibitory concentration (MIC90) for C. difficile, which typically ranges between 1 and 2 µg/mL.Reference Barlett 7 , Reference Pepin 8 Vancomycin 125 mg once daily may be sufficient as secondary prophylaxis for CDI. The duration of OVP is somewhat controversial in light of evidence suggesting that re-establishment of C. difficile colonization can occur within a few days to 3 weeks after CDI treatment.Reference Abujamel, Cadnum and Jury 9 Should OVP be stopped at the course completion of systemic antibiotic or be extended to beyond the end of systemic antibiotic, and for how long?
Many unanswered questions remain, and the interpretations of these findings are limited by the inherent nature of retrospective observational studies and conflicting results. The heightened risk period for CDI recurrence is typically within 8 weeks after CDI treatment completion;Reference McDonald, Gerding and Johnson 1 it is unclear whether case ascertainment between 90 days versus 150 days from a prior CDI contributed to the inconsistent results between these 2 studies. Furthermore, the primary end points of CDI recurrence in existing observational studies are often defined only by positive C. difficile testing with or without diarrhea, making it difficult to distinguish between colonization and actual infection in the absence of clinical assessment. Prospective data are needed to guide the selection criteria of the targeted population with the highest propensity for CDI recurrence who may benefit most from the optimal dose and duration of OVP. We await results from ongoing randomized controlled trials to further inform the clinical utility of OVP as a preventive strategy in reducing the risk of recurrent CDI. 10 , 11
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





