INTRODUCTION
Campylobacter is the most common bacterial enteropathogen in industrialized countries, Campylobacter jejuni being the most common species [Reference Blaser1, Reference Rautelin and Hänninen2]. Most infections are sporadic, although occasionally outbreaks do occur. A seasonal peak for Campylobacter infections is obvious in many countries including Finland [Reference Rautelin and Hänninen2–Reference Kovats4], where domestically acquired Campylobacter infections occur mostly during this seasonal peak. Although the source of infection usually remains unknown in sporadic cases, case-control and other studies have identified a variety of risk factors, including handling and eating poultry meat, eating raw or undercooked meat, drinking unpasteurized milk or untreated water, swimming in natural waters, contact with domestic animals, and travelling abroad [Reference Blaser1, Reference Eberhart-Phillips5–Reference Blaser, Engberg, Nachamkin, Szymanski and Blaser7].
Campylobacter infection is usually a self-limiting disease and no specific treatment is needed [Reference Blaser, Engberg, Nachamkin, Szymanski and Blaser7]. However, antimicrobial therapy may be indicated in prolonged or complicated illness [Reference Blaser, Engberg, Nachamkin, Szymanski and Blaser7] and has been shown to shorten the duration of diarrhoea in some cases [Reference Mattila8, Reference Ternhag9]. According to our earlier studies, Finnish Campylobacter patients are often hospitalized and frequently treated with antimicrobials [Reference Schönberg-Norio10, Reference Feodoroff11]. Campylobacter enteritis can cause a wide range of complications. Reactive arthritis (ReA) is reported in about 1–5% of Campylobacter enteritis cases [Reference Pope12]. Another important, although rare, Campylobacter enteritis-related complication is Guillain–Barré syndrome [Reference Tam13].
The aim of the current work was to study the incidence of complications, especially joint and musculoskeletal symptoms, reported by patients after sporadic C. jejuni enteritis of domestic origin. We were also interested in possible associations between complications and antimicrobial treatment, and the serotypes of the C. jejuni isolates.
METHODS
Subjects and questionnaire
This multi-centre cross-sectional study of sporadic domestically acquired Campylobacter infections was conducted during a seasonal peak from 1 July to 30 September in 2002. Seven clinical microbiology laboratories in the southern, eastern, western, central, and northern parts of Finland serving both rural and urban areas participated. Outpatients and hospital patients who had not travelled abroad within 2 weeks prior to illness, and whose stool culture was positive for Campylobacter were included. Patients of three of the centres participated in our previous case-control study [Reference Schönberg-Norio6].
A questionnaire concerning possible complications connected with Campylobacter infection was sent to Campylobacter-positive patients (n=406/423, 96·0%) 2 months after collection of positive stool samples. Of those patients that had participated in our case-control study, the questionnaire was sent directly to 139 that gave permission to do so and 57 further patients received the questionnaire through their physician. The remaining 210 patients from the other four study centres received the questionnaire through their physician. Seventeen (17/423, 4·0%) patients did not receive a questionnaire for the following reasons: refusal to grant permission (12 patients from the case-control study), misplaced information (four patients), and one patient who could not be reached. In addition to the questionnaires, information on Campylobacter infection was collected, with the patient's permission from medical records in 111 cases (information on antimicrobial therapy only in another 13 cases).
Campylobacter isolates
Stool isolates originally cultured in the participating laboratories were collected and stored at −70°C before analysis. C. jejuni isolates were identified on the basis of positivity for hippurate hydrolysis and serotyped as described previously [Reference Rautelin and Hänninen14] using commercially available antisera (Campylobacter Antisera Seiken Set, Denka Seiken Co., Japan) based on heat-stable Penner's antigens. Antimicrobial susceptibilities [minimal inhibitory concentrations (MIC) based on agar dilution] of the isolates have previously been described [Reference Schönberg-Norio15].
Diagnostic criteria
ReA was diagnosed based on a doctor's judgement from medical records. Synovitis was defined as joint pain with swelling or movement restriction in a previously asymptomatic joint or a clear exacerbation of joint symptoms in a previously symptomatic joint [Reference Hannu16], as reported by the patient. In this study, enthesopathy was defined as heel pain and/or pain in the Achilles tendon. Other self-reported joint symptoms connected to C. jejuni enteritis, excluding previously known joint diseases, were recorded as Campylobacter-associated joint symptoms. Extra-articular symptoms from the urinary (dysuria, frequency, discharge) and gastrointestinal tract, nervous system (nerve pain, paresthesia, n. facialis paresis), heart (chest pain, arrhythmia), skin, and eyes (pain, redness, sensitivity to light, discharge), indicating possible complications related to Campylobacter enteritis, were also recorded. Simultaneous arthritis and reactive urinary (dysuria, secretion from the urethra) and reactive eye (pain, sensitivity to light, redness) symptoms, were interpreted as being suggestive of Reiter's syndrome or incomplete Reiter's syndrome (no urinary symptoms) [Reference Keat17].
Statistical analysis
All statistical analyses were performed using SPSS version 15.0 software (SPSS Inc., USA). For categorical variables Pearson's χ2 test and Fisher's exact test were used, as appropriate. Mann–Whitney U test was used for continuous variables. Spearman's rho was used for correlations. Logistic regression was used for calculation of odds ratios (OR) and 95% confidence intervals (CI). Variables that were significant by univariate analysis were included in the multivariate model. All P values reported were two-sided, with P<0·05 considered to be statistically significant.
Ethical considerations
The Ethics Committee of the Hospital District of Helsinki and Uusimaa approved this study.
RESULTS
Questionnaire
A total of 235 (235/406, 57·9%) patients returned the questionnaire. After exclusion of the patients from one centre with an exceptionally low participation rate (17/67, 26%), the response rate was 64% (218/340). Questionnaires were somewhat more frequently returned in Kuopio (61/86, 71%) and North Karelia (25/31, 81%) compared to Helsinki (50/79, 63%), Oulu (46/74, 63%), Satakunta (14/27, 52%), and Central Finland (22/43, 51%). Results based on the questionnaire were calculated using number of positive responses out of all responses to the variable in question.
Study population
Seventeen patients who had returned questionnaires were excluded due to the following reasons: travelling abroad (eight patients), questionnaire returned blank (four patients), stool culture yielded C. coli (four patients), and verification of simultaneous Plesiomonas shigelloides infection (one patient). Thus, a total of 201 cases of C. jejuni infection were included in the final analysis. The data were representative regarding gender, age, and seasonal distribution. The median age of the patients was 50 years (range 1–88 years); of these 61 (30·3%) patients were aged ⩾60 years. Males (51·7%) and females (48·3%) were represented equally.
Symptoms
All patients had diarrhoea and 22 (11%) reported having had bloody diarrhoea. Bloody diarrhoea was more common than expected in the younger age groups (0–5 years and 6–17 years, P=0·001, Fisher's exact test). The median duration of diarrhoea was 6·0 days (range 1 to >30 days), with 22 patients (11%) reporting a duration of ⩾14 days. The majority of patients reported stomach pain (n=151, 75·1%) and fever (n=171, 85·1%), whereas nausea (41%), vomiting (10%), and headache (5%) were reported less frequently. Eighty patients (80/194, 41%) needed hospitalization due to enteritis with a median hospitalization time of 4·0 days (range 1–20 days). Fever was more common in hospitalized than non-hospitalized patients (94% vs. 79%, P=0·002, χ2 test) and duration of diarrhoea shorter (5 days vs. 7 days, P=0·04 Mann–Whitney U test).
Musculoskeletal symptoms
Joint and musculoskeletal symptoms associated with C. jejuni enteritis were reported by 39% (79/199) of patients (Table 1). The median age of these patients was 51 years (range 3–88 years). Five patients were aged <18 years (Table 1). Joint symptoms were reported slightly more often by women (43 patients, 54%) than men (36 patients, 46%) (difference not statistically significant). The most commonly reported musculoskeletal symptom was joint pain (64/72, 81%), followed by lower back pain (42/74, 53%). Approximately one-third reported movement restriction (27/72, 34%) and swelling plus warmth of joints (24/72, 30%). Symptoms suggesting synovitis (31/74, 42%) were reported in joints of upper (shoulder, elbow, wrist, hands, and fingers) and lower (hip, knee, ankle, foot, and toes) limbs (Table 1). Stomach ache during enteritis was associated with development of later joint pain (P=0·027, χ2 test).
Table 1. Musculoskeletal symptoms reported by patients in conjunction with domestically acquired C. jejuni infection during a seasonal peak in 2002

* Positive responses out of all responses to the variable in question.
† Diagnosed or suspected reactive arthritis (ReA) on the basis of medical records.
‡ Achilles enthesopathy and/or heel pain (self-reported).
§ Comprises all joints of the spinal back.
Of the reported musculoskeletal symptoms, only lower back pain was associated with gender, being more common than expected in females (P=0·013, χ2 test). Increasing age was associated with the incidence of musculoskeletal symptoms in general, showing an increase of 1·4%/year of age (P=0·045, OR 1·0, 95% CI 1·0–1·1, logistic regression, Wald's test). The specific musculoskeletal symptoms associated with increasing age were movement restriction of the joint, joint swelling/warmth, synovitis, lower back pain, and heel pain.
The median onset of musculoskeletal symptoms was 7 days (range 0–60 days) after the start of diarrhoea and the duration varied from 2 to >30 days, with almost half reporting duration >30 days.
Most commonly patients reported symptoms from large joints (55/79, 69·6%), followed by symptoms from the lower back and/or neck (53/79, 67·1%), and small joints (33/79, 41·8%). Small joints were more commonly affected in females than males (P=0·004, χ2 test). Females also reported symptoms from >5 joints more often than males (P=0·007, Fisher's exact test). Gender and age were not associated with the location of affected joints. Age was not associated with the number or size of affected joints.
Four patients were diagnosed to have ReA when visiting a physician and according to their medical records, another four had strong clinical evidence for suspicion of ReA. The peripheral arthritis was monoarticular in one, oligoarticular in three, and polyarticular in four patients (Table 1). The most frequently affected joints were ankles (six patients) and knees (four patients). The onset of symptoms was between 6 and 60 days after the start of diarrhoea (one patient reported arthritis to have started 3 days before diarrhoea). The duration of diagnosed/suspected ReA ranged from 4 days (one patient) up to >30 days (six patients), (Table 1). The median duration of diagnosed/suspected ReA together with enthesopathy, was significantly longer (31 days) than that of other Campylobacter-associated joint symptoms (10 days) (P<0·014, Mann–Whitney U test). Two patients had symptoms severe enough to be admitted to a hospital (duration of hospitalization 1 and 6 days). The severity of C. jejuni enteritis was not associated with developing ReA or enthesopathy.
A total of 18 (9%) patients (age range 3–80 years) had Achilles enthesopathy and/or heel pain, with two being children (aged 3 years and 15 years) (Table 1). Of the 79 patients reporting musculoskeletal symptoms, only 10 (10/78, 13%) patients visited a physician. Of these, 90% (9/10) reported joint pain and 60% (6/10) swelling of a joint. Of those who visited a physician, 71% (5/7) reported joint symptom duration of >30 days. Of the patients reporting enthesopathy, only one (1/18, 6%) patient (a 55-year-old female) visited a physician due to heel pain. Nine (5%) patients reported simultaneous eye symptoms (pain, redness, sensitivity to light, and/or discharge). Three (2%) patients reported simultaneous reactive symptoms from the urinary tract (dysuria with discharge or dysuria). Of these, one patient had simultaneous reactive eye symptoms. In addition, seven (3%) patients reported synovitis and all of them had reactive eye (four patients) and/or urinary symptoms (three patients), indicating possible Reiter's syndrome or incomplete Reiter's syndrome (no urethritis).
Antimicrobial treatment
Of the 201 patients included in this study, 148 (74%) received antimicrobial treatment for C. jejuni infection. Of the 148 patients, 124 (84%) received appropriate antimicrobial treatment, as interpreted from the results of antimicrobial susceptibility testing [Reference Schönberg-Norio15]. In general, appropriate antimicrobial treatment for enteritis did not affect the duration of diarrhoea (Table 2). However, for a subgroup of patients (those who participated in the previous case-control study) information on the delay from the start of diarrhoea to the initiation of antimicrobial treatment was available [Reference Schönberg-Norio6]. By dividing these particular (appropriately treated) patients into two groups, those treated within 2 days of start of diarrhoea had a significantly shorter duration of diarrhoea (median 4·5 days) compared to those who received treatment ⩾3 days after the onset of diarrhoea (median duration of diarrhoea, 7 days) (P=0·009, Mann–Whitney U test) (Table 2).
Table 2. Effect of appropriate antimicrobial treatment (as based on antimicrobial susceptibility testing, MICs, of the isolates) on domestically acquired C. jejuni enteritis and on some Campylobacter-related complicationsFootnote *
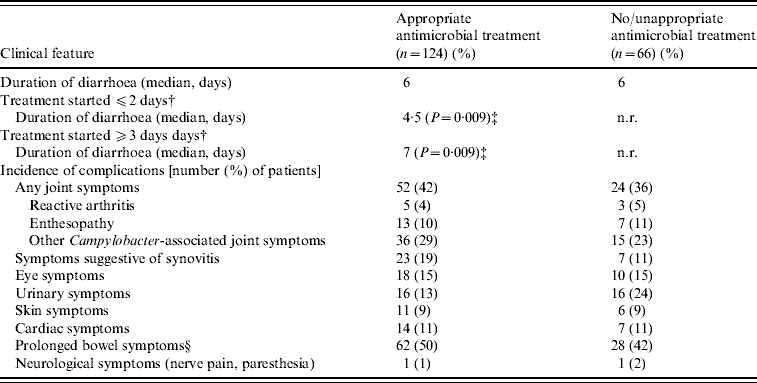
n.r., Not relevant.
* Data on antimicrobial treatment missing from 11 patients.
† This information available only for a subgroup of patients (n=58).
‡ Mann–Whitney U test.
§ Duration of diarrhoea or abdominal pain >7 days.
The median duration of antimicrobial treatment was 7 days (range 2–20 days). Fluoroquinolones were used most frequently (54/148, 37%), followed by macrolides (52/148, 35%). A combination of a macrolide and a fluoroquinolone was used in five (5/148, 3%) patients. Antimicrobial treatment was more common in hospital-treated patients than those visiting an outpatient clinic (P=0·046, χ2 test), but it did not affect the duration of hospitalization. Fluoroquinolones were used more often in hospitalized patients (P<0·0001, χ2 test) and macrolides for patients in primary health care (P=0·016, χ2 test).
The use of antimicrobial treatment for enteritis was not associated with developing any musculoskeletal complications, including ReA or enthesopathy (Table 2). Thirty-three (33/68, 49%) patients reporting musculoskeletal symptoms connected to enteritis, were treated with a fluoroquinolone for enteritis. Six patients reporting Achilles tendon pain also received fluoroquinolone for their enteritis. Appropriate treatment for enteritis was not associated with the incidence of any other complications (Table 2).
Twelve (12/201, 6·0%) patients received antimicrobial treatment 30 days prior to becoming ill and all reported one or several complications. Musculoskeletal complications were most commonly reported (nine patients: two reported enthesopathy and seven reported other Campylobacter-associated musculoskeletal symptoms). Prior antimicrobial treatment was associated with Campylobacter-associated musculoskeletal symptoms (P=0·035, χ2 test) and with developing cardiac symptoms (chest pain and arrhythmia) (P=0·046, Fisher's exact test). However, prior antimicrobial treatment had no effect on the severity of C. jejuni enteritis.
Serotypes
Thirteen different serotypes were identified in patients reporting musculoskeletal symptoms. However, no specific serotype could be linked to any reported musculoskeletal symptom or other complication. No serotype could be linked to the lack of complications either. In multivariate analysis, serotype Pen 2 (P=0·005, OR 17·4, 95% CI 2·3–129·2, Wald's test, logistic regression) and age group 0–5 years (P=0·004, OR 17·1, 95% CI 2·5–118·9, Wald's test, logistic regression) remained independent significant risk factors for bloody diarrhoea.
DISCUSSION
In this multicentre study of domestically acquired C. jejuni infections, musculoskeletal symptoms in connection with Campylobacter enteritis were commonly reported by patients (39%); the incidence of diagnosed ReA was 4% and that of Achilles enthesopathy and/or heel pain was 9%. Of all 79 patients reporting any musculoskeletal symptoms connected to enteritis, 67% had reactive Campylobacter-associated joint symptoms other than diagnosed ReA or enthesopathy and only 13% visited a physician. Of those who contacted health care, 71% reported duration of symptoms to be >30 days. Stomach ache during enteritis was connected to the later development of joint pain.
As C. jejuni is the most common bacterial enteropathogen in developed countries [Reference Blaser1, Reference Rautelin and Hänninen2], Campylobacter as a trigger of ReA is increasingly important [Reference Söderlin18–Reference Townes20]. Although the incidence of classical ReA in the current report is moderate (4%), in accord with previous reports [Reference Pope12, Reference Hannu16, Reference Schiellerup, Krogfelt and Locht21], other Campylobacter-associated joint symptoms of milder character were commonly reported. Females, previously shown to have a higher risk for ReA [Reference Townes20], reported more often than males polyarthralgia of the small joints of the hands and feet in our study. Originally, Ahvonen and colleagues [Reference Ahvonen, Sievers and Aho22], when describing Yersinia-triggered ReA in 1969 reported involvement of the small joints of the hands and feet, a finding also shown following Campylobacter infection [Reference Hannu16, Reference Kosunen23].
Enthesopathy seldom leads to health-care contact [Reference Hannu16], and in our study, only one patient with heel pain visited a physician. Stomach ache, a sign of more serious enteritis, was associated with later development of joint pain in our study. Hannu and colleagues reported stomach ache of longer duration to be associated with reactive musculoskeletal symptoms, including joint pain [Reference Hannu16]. However, we could not establish an association between other characteristics of serious enteritis (longer/bloody diarrhoea, fever, nausea/vomiting, hospitalization) and ReA, enthesopathy, or other Campylobacter-associated musculoskeletal symptoms, in contrast to some previous studies [Reference Hannu16, Reference Schiellerup, Krogfelt and Locht21, Reference Locht and Krogfelt24]. Furthermore, in our study as in some others [Reference Townes20, Reference Locht and Krogfelt24], antimicrobial treatment did not prevent reactive joint symptoms.
In the current study, antimicrobial treatment for Campylobacter enteritis was very common (73%), as recently reported from Finland and the USA [Reference Feodoroff11, Reference Townes20]. Fluoroquinolones were used significantly more often than macrolides in hospitalized patients whereas in the outpatient clinics the opposite applied. When comparing all those who received appropriate antimicrobial treatment, as based on antimicrobial susceptibility testing, to those who received inappropriate antimicrobials or no therapy at all, there was no difference in the duration of diarrhoea or later development of complications. However, a shorter delay in the initiation of antimicrobial treatment correlated with a shorter duration of diarrhoea; treatment initiation within 2 days of the start of diarrhoea, compared to treatment initiation ⩾3 days after onset of diarrhoea, shortened the median duration of diarrhoea by 2 days. This finding is supported by a recent meta-analysis, in which earlier antimicrobial treatment (within 3 days of onset of disease) was calculated to shorten the mean duration of diarrhoea by 1·73 days [Reference Ternhag9]. In hospitalized patients in our study, treatment was initiated significantly earlier and this correlated with a shorter duration of diarrhoea, compared to those not hospitalized. Although Campylobacter infection causes a significant burden of illness, antimicrobial therapy seems to have only a marginal effect on diarrhoea, even if started early and thus, should be used restrictively.
Serotype Pen 2 was independently associated with bloody diarrhoea and very young age (0–5 years). Other reports have shown bloody Campylobacter diarrhoea to be associated with young age [Reference Gillespie25, Reference Gallay26] and it is thought to reflect the frequency of colitis [Reference Blaser, Engberg, Nachamkin, Szymanski and Blaser7]. However, the incidence of long-term intestinal complications was probably underestimated in the current study since the follow-up period was only 2 months. The study was also too limited to detect rare complications like Guillain–Barré syndrome.
We obtained a satisfactory response rate of 64% (after excluding one centre) and our patient series was representative with respect to gender, age, and seasonal distribution. However, our data may be biased in that persons with milder enteritis might not have visited a physician at all and thus, this patient cohort might over-represent those with more severe enteritis or other sequelae due to C. jejuni infection. Furthermore, data relying on the patient's own observations should be interpreted with caution and it cannot be excluded that patients who have experienced enteritis are more observant, thus over-reporting joint symptoms and other complications. Medical reports, available for a moderate number of patients were of help, but could not replace the clinical assessment of the patient.
In conclusion, musculoskeletal symptoms connected to C. jejuni enteritis are common, with joint pain being the most frequent. In addition to diagnosed classical ReA occurring in about 4% of the cases, other Campylobacter-associated reactive joint symptoms are commonly observed. Although probably due to their symptoms milder nature patients seldom visit a physician and thus, these symptoms usually remain undiagnosed. Although stomach ache during enteritis may predict further development of later joint pain, the underlying mechanisms behind the frequently occurring musculoskeletal complications associated with Campylobacter infection remain to be studied.
ACKNOWLEDGEMENTS
The study was supported by the Academy of Finland (Elvira) and grants to D.S.-N. from Finsk-Norska Medicinska Stiftelsen (Finnish-Norwegian Medical Foundation), Stiftelsen Dorotea Olivia, Karl Walter och Jarl Walter Perkléns Minne, Finska Läkaresällskapet (Finnish Medical Association), and Orion-Farmos Research Foundation. The technical assistance of the late Pirjo Kosonen is gratefully acknowledged. The results of the study have been presented as part of the doctoral thesis of D.S.-N.
DECLARATION OF INTEREST
None.






