INTRODUCTION
West Nile (WN) is a notifiable vector-borne, zoonotic disease caused by the WN virus (WNV) with worldwide distribution. This flavivirus can replicate in a great number of bird, reptile, amphibian and mammal species, and has been isolated in at least 75 species of mosquitoes [Reference Hubálek and Halouzka1–Reference Sebastián3]. This wide range of potential susceptible species makes WNV the most widely spread arbovirus in the world, being present in all continents, except the Antarctic [Reference Kramer, Styer and Ebel4]. Despite this widespread presence, clinical disease has been reported only in a few species, mainly humans, equids and many species of birds [Reference Komar5, Reference Cabre6]. The role of these hosts in WN epidemiology is very diverse. Many species of birds act as WNV reservoirs, not presenting clinical signs and amplifying WNV, spread by bird–vector–bird transmission. Conversely, humans and equids may present severe clinical symptoms but are ‘dead-end’ or incidental hosts, as they are not known to develop an infectious level viraemia [Reference Komar5, Reference Sotelo7].
As an example, in the USA, where WNV was first introduced in 1999, a total of 2036 cases in equids and 10 764 cases in humans have been reported over the last 5 years (2006–2010), with an enormous sanitary and economic impact in the country [CDC database, (http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm); USDA database, (http://www.aphis.usda.gov/vs/nahss/equine/wnv/)]. Traditionally the consequences have not been so devastating in Europe, probably because the virus has been circulating for many decades (i.e. not a naive population as in the USA), and has produced sporadic and limited outbreaks in humans and equids separated by long periods of epidemiological inaction [Reference Sotelo7]. However, since 1996, WNV virulence seems to have increased, mainly in the Mediterranean basin, with an increase in the number of reported outbreaks in humans and/or equids [Reference Kramer, Styer and Ebel4, Reference Komar5, Reference Calistri8]. Example of this are the outbreaks reported in Morocco (1996–2003, 2010), Italy (1998, 2008–2009, 2010), Israel (1998–1999, 2000), France (2000, 2003–2004–2006), Portugal (2004, 2010), Greece (2010) and Spain (2004, 2010) [8, OIE-WAHID (www.oie.int/wahid-prod/public.php)].
Outbreaks of WNV in equids usually occur under specific climatic and environmental conditions and, typically, before detection of human WN cases, which could be used as an early-warning system for the public health authorities [Reference Mostashari9, Reference Ward and Scheurmann10]. Obviously, such conditions directly influence the abundance of potential vectors and the presence of amplifying birds (i.e. those birds with high levels of viraemia). The most common vector of WNV in Europe is the ornitophilic mosquito Culex pipiens. Although there are no counts of Culex spp. for European countries, it is known that its presence is mainly related to factors such as distance from aquatic areas, temperature and rainfall [Reference Tachiiri11, Reference Wimberly12]. In fact, bodies of water in which vector and bird populations are abundant constitute an ideal setting for WNV spread, and have indeed been linked with WNV circulation [Reference Calistri8].
The continuous progress in spatial remote sensing and other geospatial technologies has opened new horizons for spatial analysis. Maps containing information on climatic and environmental factors can be integrated in geographical information systems (GIS) in order to estimate suitable areas for human and animal diseases, being particularly useful for vector-borne diseases [Reference Clements and Pfeiffer13]. One of the simplest and most frequently used ways of combining input (or factor) maps to produce suitability maps is weighted linear combination (WLC) [Reference Makropoulos and Butler14]. This method allows the combination of several input or factor maps and the assigning of weights to account for the relative importance of each factor. The basic steps used in WLC are (1) to standardize the scores of the input maps and compare all these layers on a common scale, (2) to apply weights to input maps and (3) to obtain the final risk map by calculating a weighted average across factors for each 1 km2 pixel. This GIS-based framework with WLC has been previously used, for example, to identify high-risk areas for Rift Valley fever in Africa [Reference Clements, Pfeiffer and Martin15]. In the current study we will use this approach to explore highly suitable areas for WNV outbreaks in equid populations.
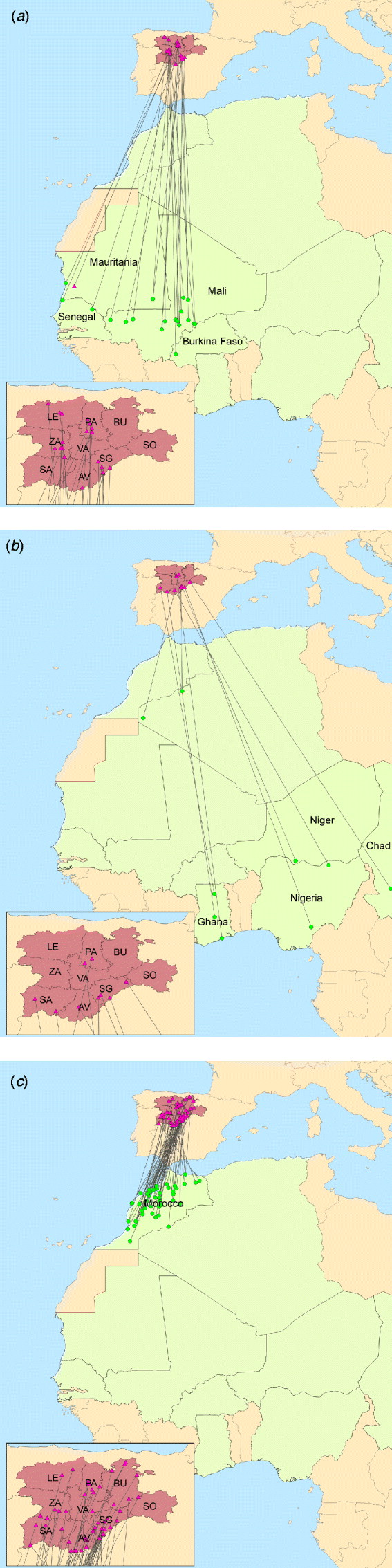
The climatic and biogeographical characteristics of Spain allow a great diversity of distinct ecosystems and biological habitats, which favours not only the presence of a great number and biodiversity of vertebrates, especially birds, both resident and migratory, but also a high abundance of mosquitoes. In fact, the southerly position of Spain, in the Palaearctic, favours the reception of a large bird population that migrate from Europe to Africa and vice versa, acting as a wintering quarter for many North European aquatic birds and as an important northern breeding area for other birds like raptors and Passeriformes, which winter in Africa. Some of these birds are able to migrate not only from Morocco, where WNV outbreaks in equids were notified in 2010 (OIE-WAHID), but also from sub-Saharan locations such as Senegal where WNV is endemic [Reference Cabre6, Reference Chevalier16] (Fig. 1). These factors, together with the re-emergence of WNV in neighbouring countries such as Portugal or France, the recent outbreaks of WNV in southern Spain, the experience gained with other vector-borne diseases such as bluetongue and, the high density of equids in certain regions of Spain has increased the concerns of the Spanish Animal and Public Health authorities regarding the potential sanitary and economic consequences of a WNV incursion into Spain. Specifically, Castile and Leon (C&L), located in the north-central part of country, is the largest region in Spain and hosts favourable conditions for the establishment of WNV, as well as a large and valuable population of equids. In fact, C&L is the third largest producer of equids, concentrating 12·19% of the total equid population of Spain [INE database (http://www.ine.es)].
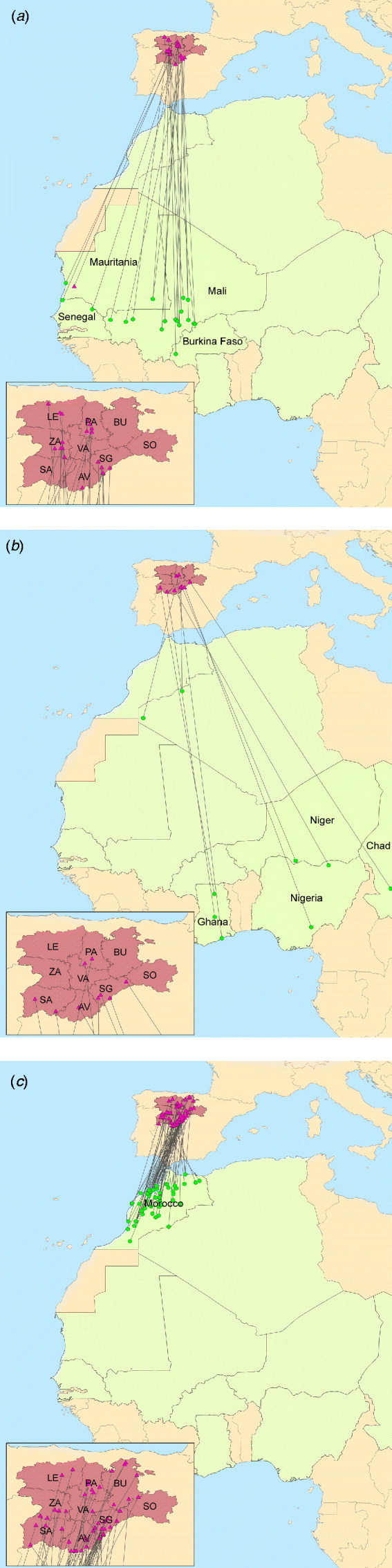
Fig. 1. Castile and Leon – Africa band records of wild birds. Triangles denote banding locations. All locations, except for one in Mauritania, are in the different provinces of Castile and Leon: Avila (AV), Burgos (BU), Leon (LE), Palencia (PA), Salamanca (SA), Segovia (SG), Soria (SO), Valladolid (VA) and Zamora (ZA). Dots denote band recovery locations, mainly in (a) African western countries, (b) African eastern countries and (c) Morocco. [Source: Spanish Office of Migratory Species (OEM, MARM) and Animal Health Research Centre (CISA-INIA).]
Although WNV infection in Spain has been suspected since 1979 [Reference Filipe and De Andrade17], its presence has been confirmed in the laboratory only recently. WNV has been identified in Spain in humans, birds and equids. Human cases corresponded to three hospitalized men, one in 2004 [Reference Kaptoul18] and the others in September 2010 [19]. Bird cases, in which WNV RNA was detected, were found in two emblematic species of Spanish predatory birds (Aquila chrysaetos and Hieraaetus fasciatus) in central Spain during 2007 [Reference Jiménez-Clavero20], while several other species of migratory and resident birds had seropositive results in southern Spain in previous years [Reference Figuerola21–Reference López23]. Finally, the first outbreak in equids was declared in September 2010 in the province of Cadiz (Andalusia), followed in less than 2 months by 27 additional outbreaks in the same province, two in Seville and one in Malaga (Andalusia) (OIE-WAHID). The total number of equids affected was 39 (out of the 845 equids at risk) (OIE-WAHID). The National WN Surveillance Plan, which has been ongoing since 2001, includes the application of active and passive surveillance methods in equids (sampling in areas close to wetlands and investigation of WNV-compatible cases, respectively) and birds (sentinel and wild bird sampling and investigation of abnormal increase of mortality, respectively), as well as entomological surveillance. Although these surveillance strategies focused mainly on areas close to the most important wetlands of Spain, the cost-effectiveness of the surveillance programme could be optimized if new strategies were mainly allocated to suitable areas for WNV. Considering the marked consequences of WNV in equids and humans, a highly sensitive surveillance system is highly recommended.
Therefore, the goal of the study presented here was to identify suitable areas for WNV outbreak occurrence by using a GIS-based framework at a high geographical resolution in one of the most important regions of Spain in terms of equid production, i.e. C&L. Methods and results presented here may guide risk-based surveillance systems for WNV in C&L or other Spanish or European regions with similar conditions and, ultimately, may help to prevent future outbreaks in equid and human populations.
METHODS
GIS-based WLC was performed in three analytical steps: (1) identification of risk factors related to WNV suitability, data gathering and generation of spatial maps for each risk factor, (2) generation of the WNV suitability map and (3) sensitivity analysis.
Identification and mapping of risk factors related to WNV suitability
A systematic review of the published literature was performed in electronic resource databases (PubMed, ISI Web of Knowledge) combining several terms such as ‘West Nile’ and ‘risk factors’, ‘West Nile’ and ‘temperature’, ‘West Nile’ and ‘climatic conditions’, ‘Culex’ and ‘temperature’ and so on, and all references found were stored in EndNote®. Hence, five risk factors for WNV suitability were identified and mapped in a raster format of 1 km2 cell size: distance from aquatic areas, temperature, rainfall, presence of wild birds, and equid density.
To the best of the authors' knowledge, distribution maps of Cx. pipiens have not been published until now. However, many studies have described the climatic and environmental variables that could favour an increase in Culex spp. populations. The first factor to be considered is the distance from aquatic areas. Almost all WNV outbreaks in Europe have occurred near to water areas, especially in deltas with abundant populations of birds and mosquitoes that interact with human and equid populations [Reference Hubálek and Halouzka1]. In fact, we estimated that the 205 OIE-notified European and Mediterranean outbreaks that occurred from 1999 to 2010 were at a mean distance of 3·2 km (95% confidence interval 0·2–11·7 km) from the main aquatic areas. The presence of aquatic habitats seems to be the most influential factor for vector survival and activity mainly because the first three stages of Culex spp. development (egg incubation, larva development, pupa development) occur in such areas. Even when these mosquitoes become adults, their flying range is limited to an area of movement not further than 7 km from aquatic areas, preferentially at 2 km [Reference Tachiiri11]. For our study, we measured the Euclidean distance to each hydrological feature in ArcGIS maps (rivers and their tributaries, lakes, reservoirs and wetlands; information obtained from ESRI 2005), assuming that the closest areas to the aquatic features have the highest suitability for Culex spp. and the value decreases linearly up to 7 km. Areas beyond 7 km were accordingly excluded.
Another important factor for Culex spp. suitability is temperature. Vector survival, breeding, feeding and development are affected by temperature [Reference Tachiiri11, Reference Ward24]. High temperatures (30–32°C) promote faster mosquito maturation and a decrease in the virus incubation period in mosquitoes, although extremely high temperatures (above 35–40°C) may prove fatal. Bearing in mind that Culex spp. mosquitoes live only 2 weeks, the faster the virus develops, the greater the likelihood of transmitting the virus to a susceptible [Reference Epstein25–Reference Reisen, Fang and Martinez27]. Conversely, low temperatures (14–18°C) cause a decrease in the mosquitoes' metabolic activity, flight and feeding behaviour [Reference López-Vélez and Molina Moreno26, Reference Mellor and Leake28]. As a consequence, optimal temperature conditions for the presence of Cx. pipiens in C&L only occur during April–October. This period was established based on the knowledge that the minimal temperature that allows viral replication in vectors is estimated to be 14°C [Reference Reisen, Fang and Martinez27].
Rainfall rate is another factor that has been positively associated with abundance of Culex spp., although the relationship is not so clear [Reference Tachiiri11, Reference Wimberly12, Reference Chevalier16] as it may be influenced by local factors, such as topography, type of soil or vegetation. These parameters directly impact the soil capacity of creating stagnant water, which is the preferred habitat of mosquitoes.
Temperature and rainfall rates were obtained from the Agrarian GIS MARM database [29]. The data consisted of the monthly mean temperature and rainfall rates from April to October during the last 50 years, gathered from each of the 218 and 754 point locations where temperature and rainfall climate stations were situated in C&L, respectively. Raster maps for temperature and rainfall were obtained by using the inverse distance weighted algorithm for each month at risk.
Regarding the presence of wild birds in Spain, it is known that the arrival of breeding migratory birds from Africa, that may have spent the winter in infected areas, occurs primarily in spring, although depending on the species and latitude they may arrive sooner or later. Such birds remain in Spain until the beginning of autumn, coinciding with the first arrivals of the wintering birds from northern Europe. This range of time, between spring and autumn, coincides with the time at which WNV outbreaks tend to occur in the Mediterranean basin (July–September), which is also favourable for the increase in mosquito populations [Reference Calistri8]. The highest seroprevalences in these summer migratory birds and resident species has also been described by Figuerola et al. [Reference Figuerola22].
We used four criteria to select the bird species that most contribute to the suitability of WNV in C&L: (i) relation to WNV outbreaks, (ii) period of the year, (iii) habitat and (iv) abundance. The first criterion refers to whether or not the wild bird species were likely to come from countries with WNV outbreaks. Currently, WNV circulates in many European countries and the Mediterranean basin. However, the highest prevalences have been found in sub-Saharan countries, from which many species of birds fly to Spain to breed [Reference Figuerola22]. Given this criterion, breeding species were selected from the migratory birds from the bird information guide of C&L [Reference Sanz-Zuasti and Velasco30]. Second, we selected only those species that were present in large numbers during the maximum estimated period of vector abundance.
Given the clear link between WNV and aquatic environment, we selected only birds associated with an aquatic habitat (wetlands, rivers, riversides, riverside forests, reservoirs) from the bird information guide of C&L [Reference Sanz-Zuasti and Velasco30], and also considered breeding and resident species. The last criterion used was abundance. Given the apparent lack of specificity of WNV to certain bird species, and considering that high density of birds may contribute to the amplification and spread of WNV, we selected all bird species considered to be abundant (i.e. more than 2000 pairs) from the bird information guide of C&L [Reference Sanz-Zuasti and Velasco30]. This criterion included resident as well as breeding birds in C&L with wintering quarters in North and sub-Saharan African countries. According to these four criteria, from a total of 199 bird species, 32 were selected (Table 1) and their distribution areas obtained from the MARM database [31]. For each bird species, a raster map was generated with values 1 or 0 in each cell, designating presence or absence of the specific bird in its nesting zones, respectively. Then, the 32 maps were joined by summing their presence/absence value.
Table 1. Breeding and resident wild bird species for WNV outbreak occurrence in Castile and Leon
The last risk factor considered for WNV suitability was equid density [Reference Ward24, Reference Murgue32]. Equids and humans are highly susceptible to WN disease, but usually outbreaks in equids are notified earlier than outbreaks in humans, mainly because equids are more exposed to vector bites. Moreover, vectors may be attracted by areas with a high density of equids because of the high excreted volumes of CO2. Location of the 9423 equid premises and census (i.e. number of equids on premises, ranging from 1 to 2050 animals) were provided by the Regional Government of C&L. We used a kernel density function to create a raster map of equid density in the C&L region of Spain.
WNV suitability map
Once the raster maps for each risk factor were created, we performed a standardization process to provide a unique comparable scale that allowed the combination of each risk factor before producing the suitability map. The method used for standardization is referred to as linear scaling [Reference Drobne and Lisec33] and uses the following formula:
where x i denotes the index value of each risk factor x for each cell I; R i is the raw score of the factor x for the cell i; R min is the minimum score (except for temperature where the minimum degrees for virus replication in the vector were considered as 14°C); R max is the maximum score and SR is the standardized range, 255. The index value (x i ) will consequently range between 0 and 255. We used this byte scale, which has been recommended by Drobne & Lisec [Reference Drobne and Lisec33] because it optimizes the computation for geo-statistical analysis. Suitability maps are presented on a graduated blue/green/yellow/orange/red scale, ranging from 0 to 255. The final WNV suitability maps were rescaled to 0–1 to facilitate interpretation.
Weights for each risk factor were given based on available literature and expert opinion and combined by using the WLC. This method is based on the following equation:
where R i is the aggregation result for each cell i, w j is the weight of the risk factor j and x ij is the value of the cell i for the risk factor j, assuming that there are a total number of n risk factors.
Specifically for this framework, the WLC applied was:
where R si is the aggregation result for each cell i of the WNV suitability map; R ei is the value for each cell i of the equid map; R wbi is the value for each cell i of the wild bird map; R cpi is the value for each cell i of the Cx. pipiens map. The latter was estimated as:
where R dhi is the raster value of the distance from humid areas map; R ti is the value of the temperature map and R rri is the raster value of the rainfall rate map.
The ‘weighted sum’ function of the ArcGIS 9.2 (ESRI, 2006) software was used to perform the analysis for each one of the 94 243 cells registered in C&L for each risk factor and to draw the final WNV suitability map. Areas from low (0) to high (255) WNV suitability in C&L were represented using colours from blue to red, respectively.
Sensitivity analysis
All modellers are aware that the weight applied to each factor to obtain the suitability map is the most critical (and subjective) aspect when using WLC. For this reason, we performed an extensive sensitivity analysis to evaluate the influence that changes on the relative weights of the input factors could have on model outcomes. The analysis was performed by systematically increasing and decreasing the weights for each of the input maps by a total of 25% of their initial value. In this way, a total of 10 different scenarios were tested. We used non-parametric Spearman correlation coefficients and box-plots to compare the outputs obtained in the reference scenario (see first three subsections of the Methods section) with the outputs obtained in each of the 10 new experiments. A change of <10% in the suitability map scores and on correlation coefficients, as well as overlapping of the notches of the box-plot was considered evidence of the robustness of the model to variations in weights. R-language (v. 2.11.1, R Foundation for Statistical Computing, Vienna, 2010) was used to perform the analysis.
RESULTS
Risk factor suitability maps
Equid density, wild bird presence and occurrence of environmental variables favourable for the establishment of mosquito populations were not homogeneously distributed in the Spanish region of C&L (Fig. 2).
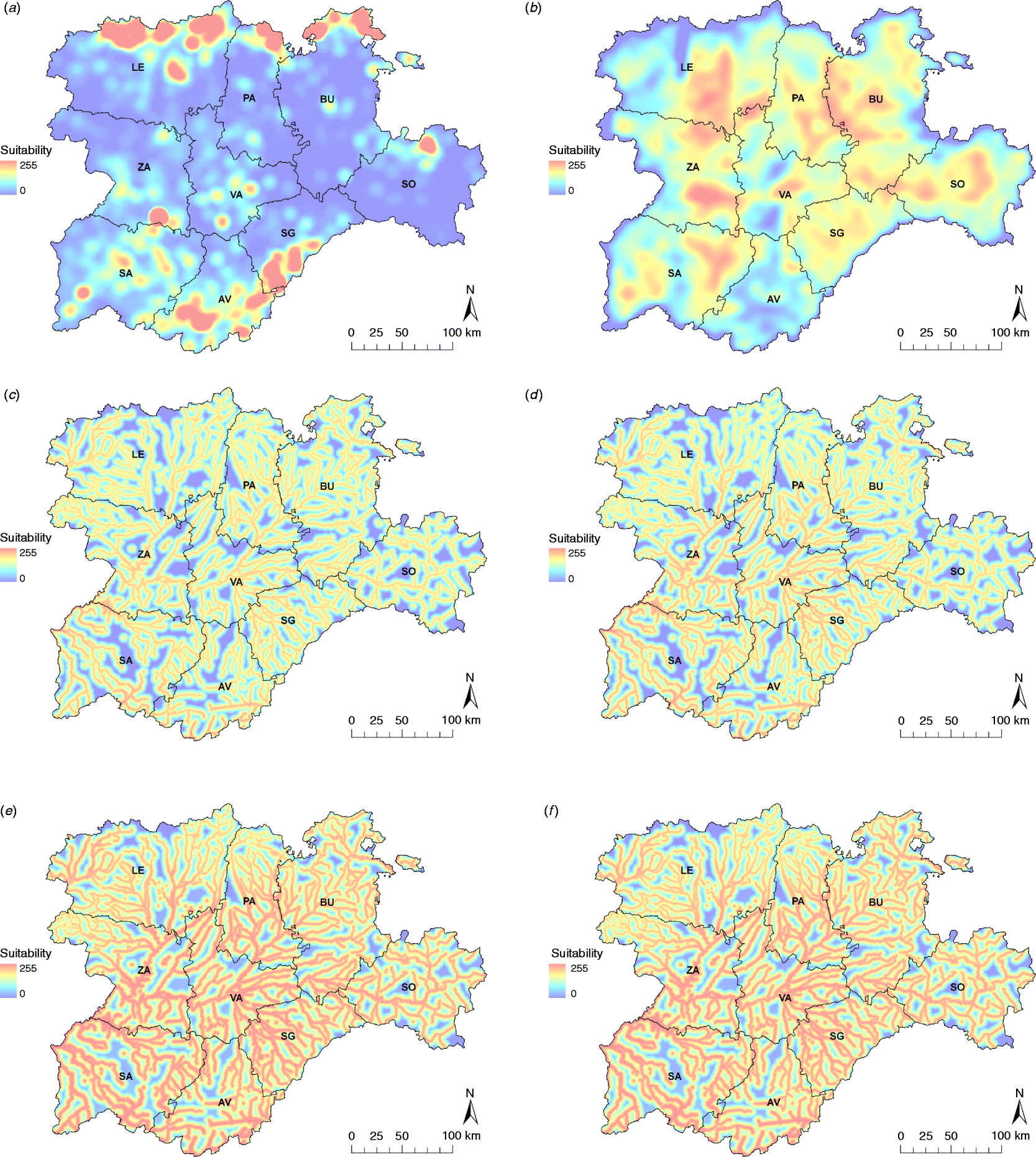
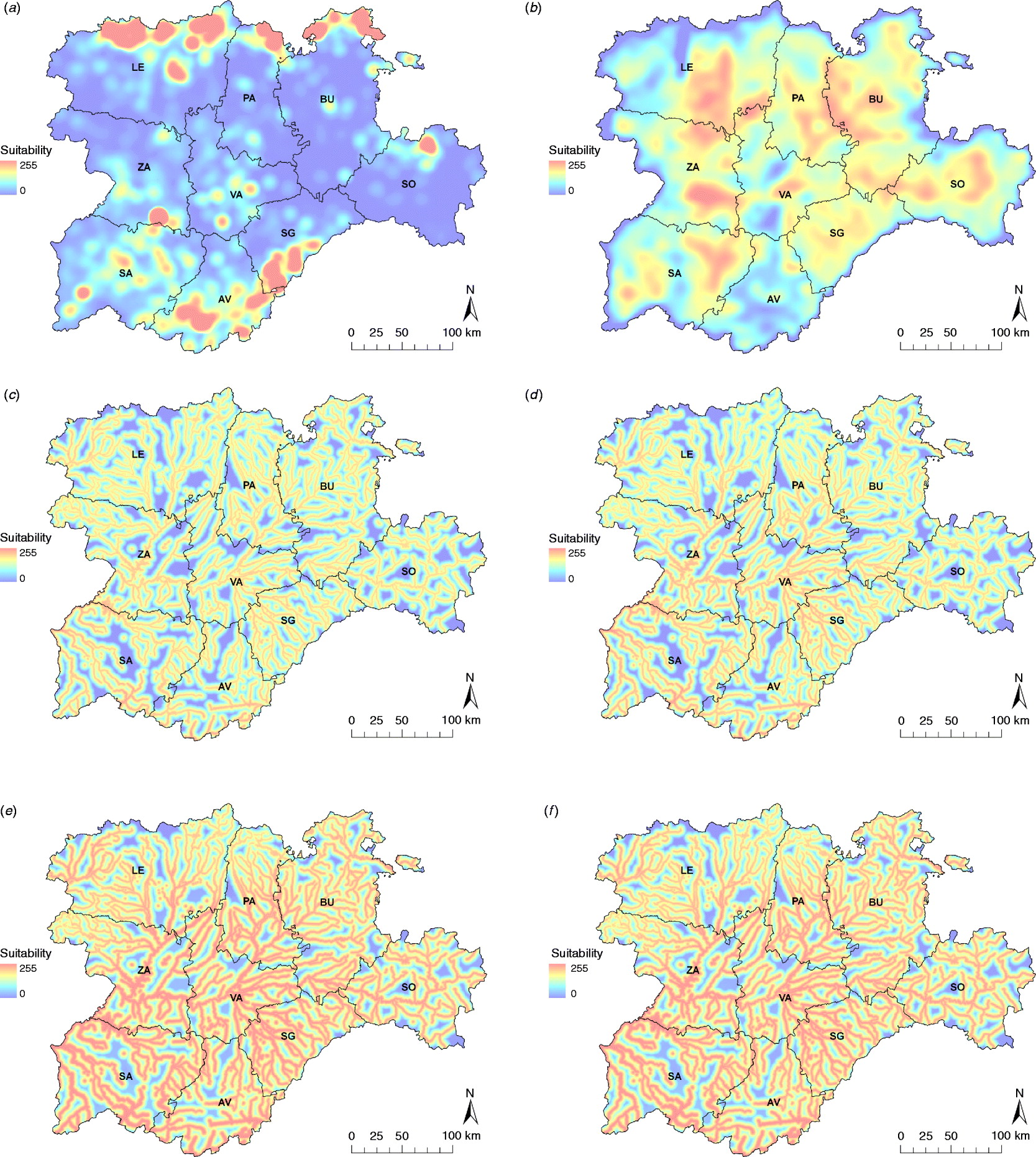
Fig. 2. Input risk maps used to obtain the WNV suitability map: (a) equid density map, (b) wild bird species abundance map and Cx. pipiens maps, (c) for the whole risk period, and for the most suitable months, i.e. (d) June, (e) July, (f) August and (g) September. Provinces of Castile and Leon are Avila (AV), Burgos (BU), Leon (LE), Palencia (PA), Salamanca (SA), Segovia (SG), Soria (SO), Valladolid (VA) and Zamora (ZA).
Equid density was mainly concentrated in the northern strip of Leon (LE), Palencia (PA) and Burgos (BU), and the southern area of Segovia (SG) and Avila (AV) in the Central System region. Other equid-dense areas can be found in Soria (SO), Zamora (ZA) or Valladolid (VA). Similarly, the wild bird abundance map represented the biodiversity of migratory and resident breeding birds. In the eastern half of the region, the map showed a more uniform wild bird distribution, highlighting some areas (especially in PA-BU and SO). In the western provinces, such as LE, ZA and Salamanca (SA), important suitable areas were identified, whereas the rest of the region was less suitable.
The suitability of Cx. pipiens, which was obtained by combining the distance from the aquatic areas map with the temperature and rainfall maps, was higher in the southwest part of the region (Fig. 2), with July and August the months with the greatest potential to host Cx. pipiens populations (Fig. 3).
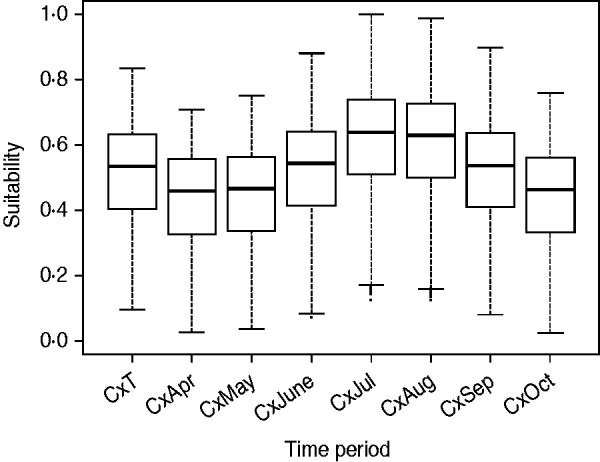
Fig. 3. Suitability for Cx. pipiens for each raster cell during the whole risk period (i.e. CxT) and for each of the seven months [from April (CxApr) to October (CxOct)].
WNV suitability map
Zones with the highest suitability for WNV outbreaks were concentrated in the Guadarrama mountains (south of SG and a small part of AV) and the Cantabric mountains (north of LE, PA and BU) (Fig. 4). Some other suitable areas were located in SA, south of ZA, north of SO and the centre of VA, the majority coinciding with important riverbeds (Fig. 4).
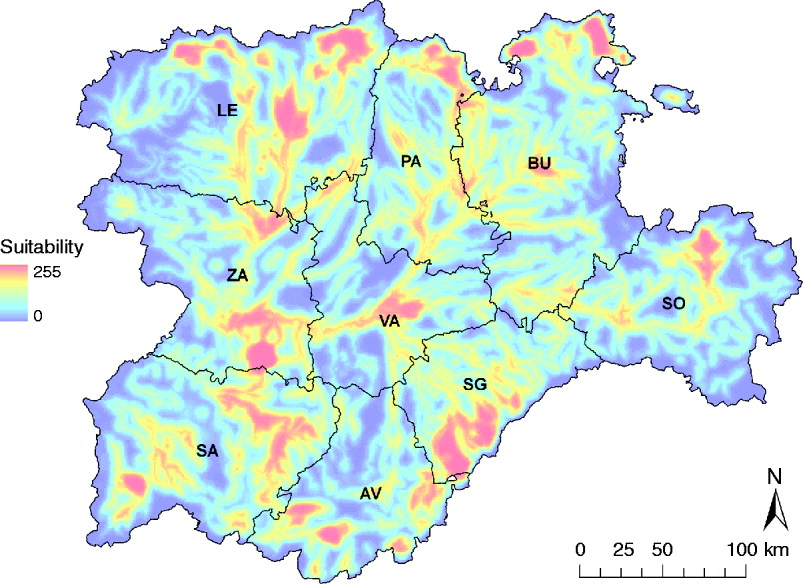
Fig. 4. WNV outbreak occurrence suitability map in the Castile and Leon region of Spain considering the whole risk period, from April to October. Provinces of Castile and Leon are Avila (AV), Burgos (BU), Leon (LE), Palencia (PA), Salamanca (SA), Segovia (SG), Soria (SO), Valladolid (VA) and Zamora (ZA).
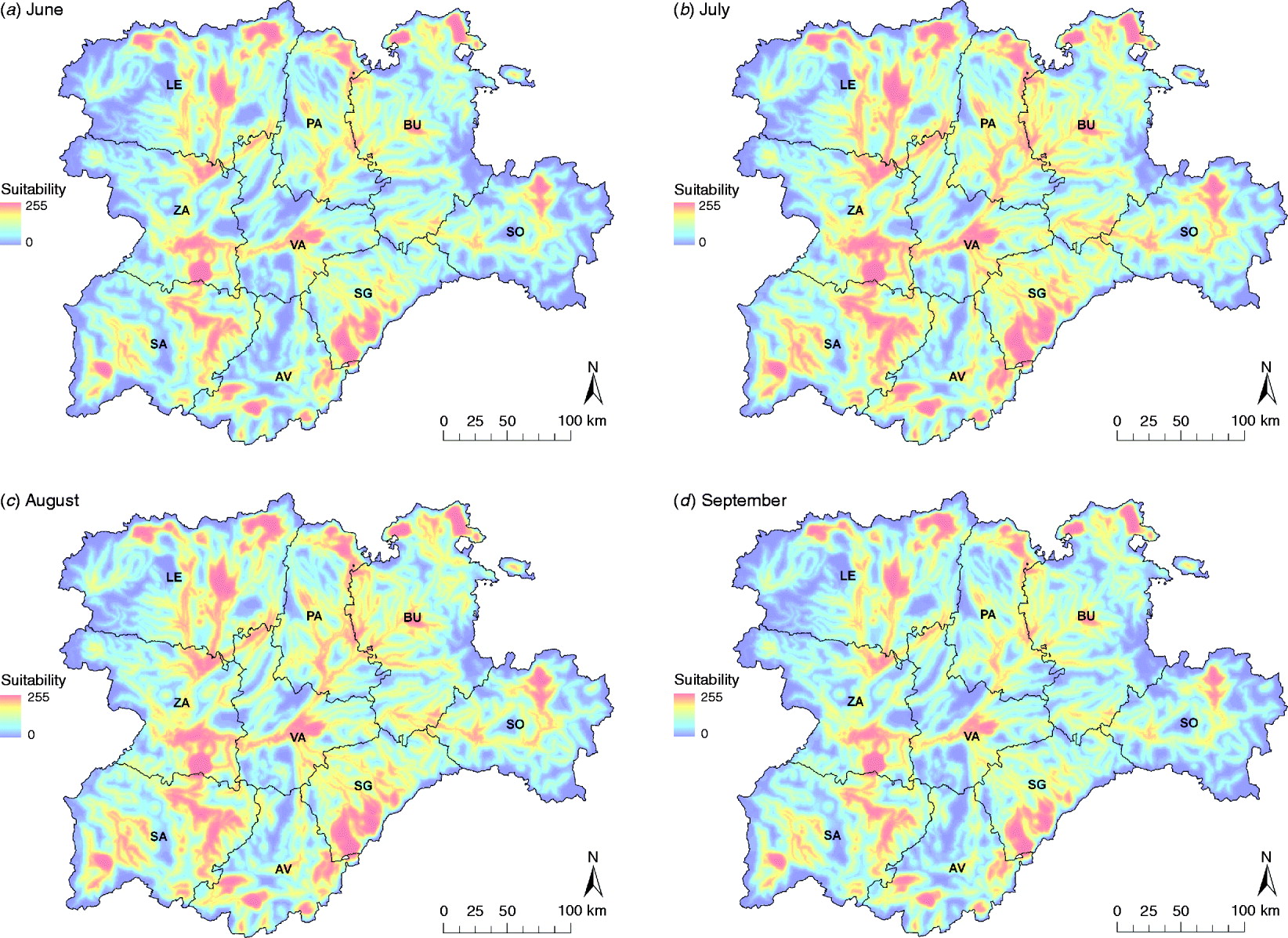
Summer months were the most suitable for a WNV outbreak occurrence, mainly due to the highest presence of Cx. pipiens, specifically July and August (Fig. 5). The main suitable spots remained for the whole period, but during July and August suitability scores increased in these areas and their surroundings.
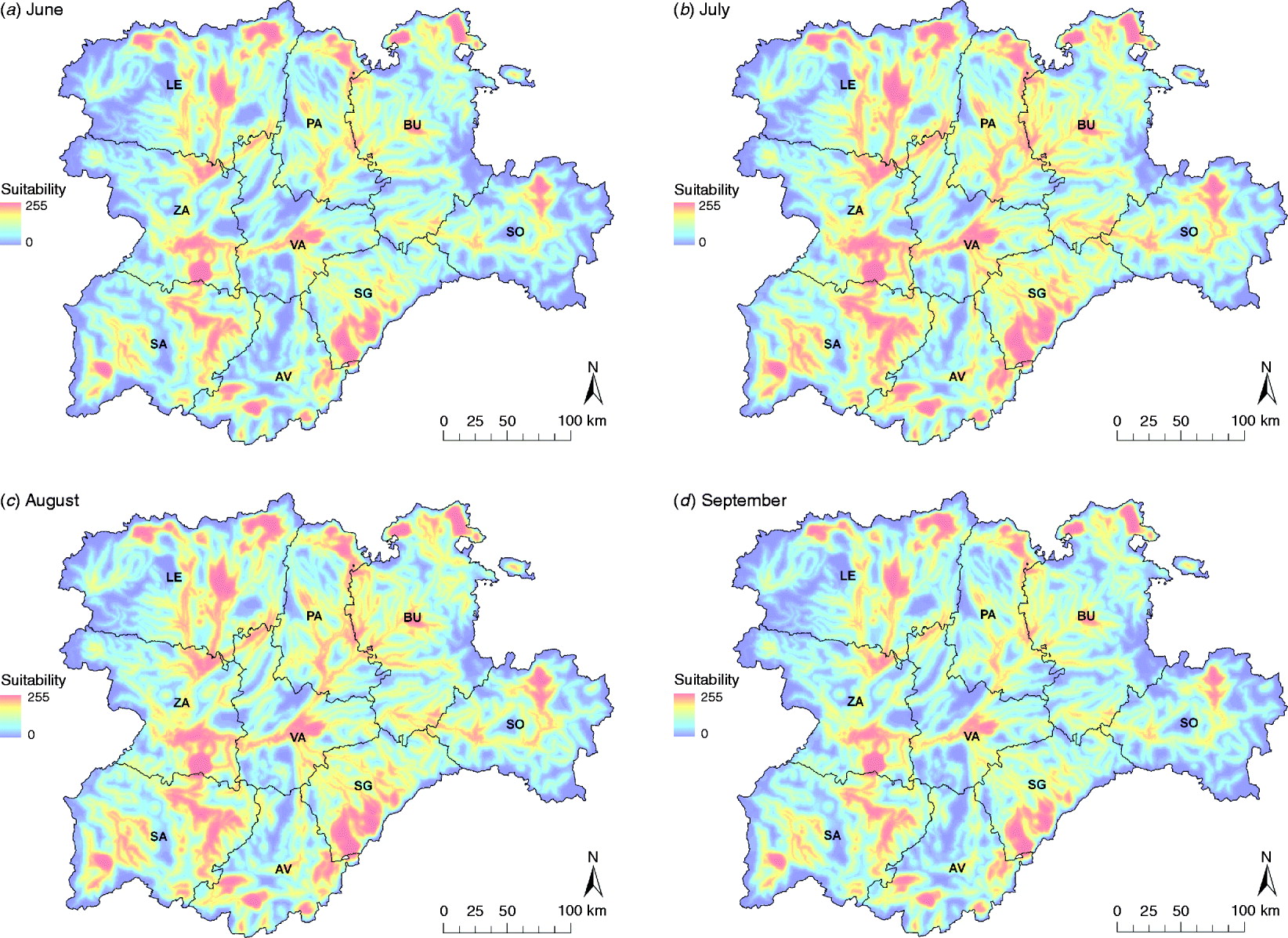
Fig. 5. WNV outbreak occurrence suitability maps in the Castile and Leon region of Spain of the summer months, from June to September. Provinces of Castile and Leon are Avila (AV), Burgos (BU), Leon (LE), Palencia (PA), Salamanca (SA), Segovia (SE), Soria (SO), Valladolid (VA) and Zamora (ZA).
Sensitivity analysis
There was <10% change when comparing the overall scores of the WNV suitability maps obtained for the reference scenario with those obtained for each of the 10 alternative scenarios. Furthermore, we did not find differences in the distribution and values (box-plots overlapping and Spearman rank correlation not lower than 0·988) when comparing each cell of the suitability map obtained for the reference scenario with those obtained for the 10 alternative scenarios (Fig. 6). Consequently, model results were considered to be robust and not sensitive to changes in the relative weights of the input risk factors.
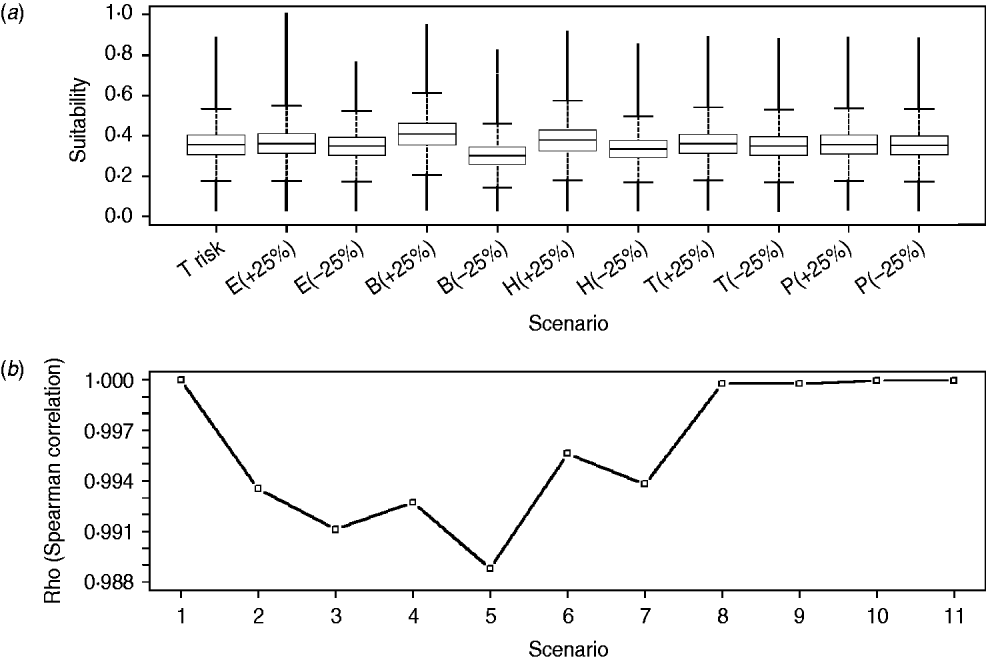
Fig. 6. Distribution of WNV outbreak occurrence suitability scores for raster cells of the reference scenario (i.e. T risk) and each of the scenarios with changes on the relative weights of the input risk factors (i.e. alternative scenarios) (a) and Spearman correlation coefficients (Rho) between raster cells of reference scenario and of every alternative scenario (b). E, Equid density; B, wild bird density; H, distance to humid areas; T, temperature; P, rainfall; (+25) and (–25) represent the increase and decrease on the relative weights for each scenario, respectively. Scenarios 1–11 in panel (b) correspond to combination between the reference scenario (T risk) and every alternative scenario represented in panel (a) [1, T risk vs. T risk; 2, T risk vs. E(+25); 3, T risk vs. E(–25), etc.].
DISCUSSION
This paper presents the first WNV suitability map in a European context. The application of a GIS-based WLC framework together with information on risk factors for WNV outbreak occurrence such as equid density, wild birds and Cx. pipiens potential habitat, allowed us to estimate the main areas for WNV suitability in the largest and one of most important regions for equid production in Spain.
The regions where WNV outbreaks were more likely to occur were mainly located in the northern and southern parts of C&L, which have high density of both equids and wild birds. Most of these locations, which were mainly distributed along the mountain chains that surround the region, are designated as Important Bird Areas (IBAs). The IBAs are recognized areas where bird biodiversity is specially protected and that concentrate exceptionally large numbers of migratory or congregatory species. Many of these relevant IBAs are very close (or include) important hydrological features, such as the Tietar valley, in AV, and the reservoirs of Riaño, in LE, or Aguilar de Campoo, in PA. The great biodiversity found in the IBAs together with the presence of competent vectors could favour the circulation of WNV in these areas, as hypothesized in other regions with similar conditions [Reference Durand34]. Moreover, WNV suitability was found to increase in the summer months. This is in agreement with the seasonal variation described for Cx. pipiens [Reference Figuerola22, Reference Malkinson35, Reference Ponçon36] as well as with the increase of WNV outbreaks historically registered in the Mediterranean basin [Reference Calistri8, Reference Zeller and Schuffenecker37, Reference Sánchez-Seco and Navarro38]. If we consider that outdoor equid activities are popular and frequent in the surroundings of IBAs, and occur mainly in summer time, the potential exposure of equids to mosquito bites is likely to occur.
Historically, the combination of spatial factors by WLC has been used to estimate disease suitability in the absence of more detailed epidemiological datasets both in developed or developing countries [Reference Clements and Pfeiffer13]. It also serves as a good approximation to identify high-risk areas where more specific studies can be conducted in the future. However, it should be noted that general environmental and climatic factors are not always good indicators of vector distribution, as there are micro-climatic conditions that may determine an increase of the presence/abundance of vector populations and that are difficult to incorporate into the model. It is clear that there is an evident need to quantify the abundance and distribution of the competent vectors, not only for the evaluation of WNV but also for other vector-borne diseases. In Spain, for example, the entomological surveillance of bluetongue has yielded very useful information to better prevent and control the disease [e.g. 39]. It would be very useful for WNV surveillance to improve the Cx. pipiens abundance map presented here for the whole Spanish territory. Following the results presented in this study, the trapping and estimation of Cx. pipiens abundance could be prioritized in the areas and time periods identified as highly suitable for WNV outbreaks, and, if financial and personal resources are available, other regions or time periods of Spain could also be studied.
One of the important limitations of the GIS-based WLC method is the potential subjectivity associated with the selection and weighting of risk factors, which could certainly bias the results obtained. In this paper, we performed an intensive review of published scientific literature and an expert opinion elicitation in order to better select and weight the risk factors. Due to the potential bias associated with the weighting scores, sensitivity analysis is a necessary procedure to assess how changes in weights can impact model outcomes. The results of the sensitivity analysis showed that model results were not sensitive to changes in weighting scores, which supports the robustness of the model outcomes (Fig. 5).
Since the real and complete epidemiological cycle of the virus is not completely understood, the model may be overlooking some relevant aspects of the reality of the disease. Some improvements of the model, mainly related to spatio-temporal variations of the risk factors, may be incorporated in the future if information becomes available. As an example, the distance from aquatic areas map represents the limitation of a static measure, which means that aquatic areas are assumed not to change during the period at risk, remaining full even in the driest months. Similarly, the wild bird map represents the distribution (i.e. nesting areas) of the selected bird species in certain locations. However, birds can move easily and this spatial variation is difficult to incorporate into a map. This problem was partially solved by using nesting zones instead of bird observations, which consider that bird populations in that moment are more sedentary, therefore this was recognized as the best available estimator. Moreover, although resident and breeding bird species warrant the main interest, other species could be considered, particularly wintering species. Wintering birds, which are mainly aquatic birds, arrive in Spain as early as August, and thus, can easily have contact with mosquitoes. These birds could move through short migrations from Mediterranean regions to C&L even in a single day, reaching the most suitable areas when temperature is favourable for a WNV outbreak occurrence. Furthermore, it is known that many migratory birds move from European countries to C&L, and vice versa. In particular, there are bird movements registered between Spain and certain European areas such as northern Italy, where the virus periodically recirculates (OIE-WAHID). Subsequently, these species should be added to future work. Nevertheless, considering that C&L is included in the Atlantic migratory routes, which originate in northern European countries where WNV has not currently been detected, that temperatures in C&L can be very low at the end of summer, and that no information was readily available to accurately map the presence of those birds, wintering birds were not considered in this analysis.
One of the main advantages of the methods and results presented here is that they can be directly used to optimize the allocation of sanitary resources of future risk-based surveillance and control programmes for WNV in the C&L region. For example, there are some suggested improvements for application in the current WN surveillance programme in C&L, which are mainly based on the active sampling of equids, taking place during August and September. First, the allocation of equids to be sampled, which is currently selected based only on the abundance of wild birds, should also consider the species of wild birds at high risk for WNV suitability and the potential presence of Culex spp., which is reflected in the WNV suitability map presented in this study (Figs 4 and 5). In fact, according to our model, only 38 (45·78%) of the current sampling locations in the C&L surveillance programme were estimated to be suitable for WNV outbreaks (Fig. 7). Furthermore, July, a month not currently included in the surveillance plan, concentrates the highest suitability, which reflects that sampling strategies should begin earlier, not in August as currently programmed. All these strategies are aimed at the early detection WNV which, ultimately, will help to rapidly control the infection and to prevent further spread of WNV into the region.
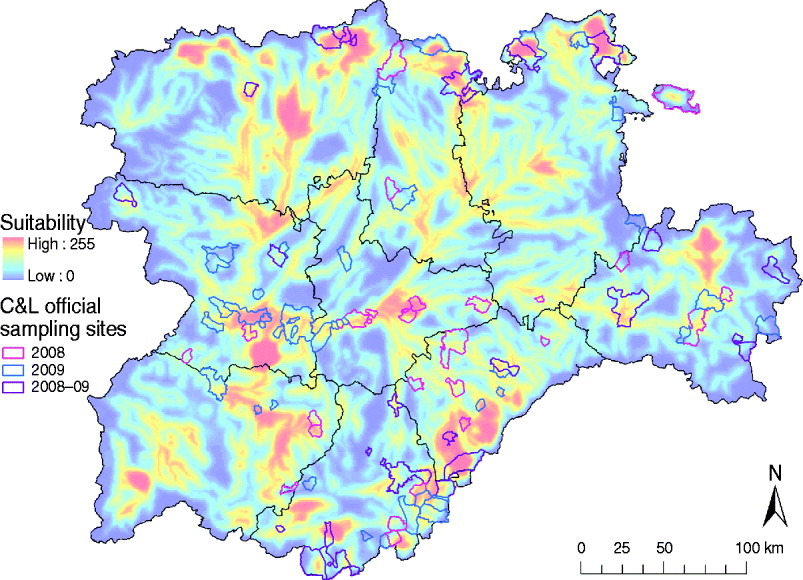
Fig. 7. Overlay of the distribution of the main sampling areas for wild birds and equids of the 2008 and 2009 WN Surveillance Plan for Castile and Leon and the WNV outbreak occurrence suitability map of Castile and Leon. Sample municipalities were obtained from the Regional Government of Castile and Leon region.
The identification of suitable areas for WNV would also help to prevent WNV epidemics in human populations. Only 61/2248 municipalities in the C&L region have a population density >100 habitants/km2 (INE database), but 40 (65·57%) of those densely populated municipalities are in areas identified as highly suitable for WNV outbreaks (Fig. 8). These municipalities should be targeted in public health surveillance and training programmes to rapidly detect any WNV incursion which can affect human populations.
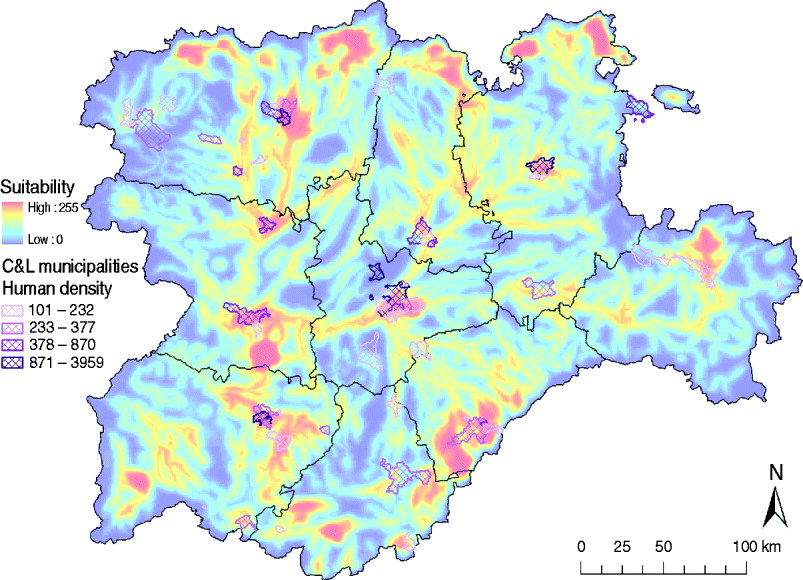
Fig. 8. Overlay of the high human density areas (>100 habitants/km2) and the WNV outbreak occurrence suitability map of Castile and Leon.
Although many uncertainties exist, several mechanisms such as enzootic transmission throughout the year in temperate regions, the vertical transmission described in some vectors or the overwintering of infected mosquitoes, may contribute to the endemicity of WNV into a country or, at least, the maintenance of the infection during the inter-epizootic periods [Reference Komar5, Reference Calistri8]. These critical aspects, together with the re-emergence and increasing severity of WNV cases into the Mediterranean area in recent years, pose a considerable threat to Spain. The identification of suitable areas for WNV outbreaks presented in this study may facilitate the implementation of risk-based surveillance systems to better prevent and control disease in equid and human populations.
ACKNOWLEDGEMENTS
The project was funded by an FPU grant of the Ministry of Education and a collaboration agreement, TRAGSA (367/2008), between the Regional Government of Castile and Leon (JCyL) and the Complutense University of Madrid. We gratefully acknowledge the assistance of Olga Mínguez and her team (JCyL), as well as Irene Iglesias and Jaime Bosch (CISA-INIA), for providing data and assistance in the interpretation of the results.
DECLARATION OF INTEREST
None.