INTRODUCTION
Varicella is a highly infectious disease caused by the varicella-zoster virus (VZV) [Reference Gershon, Takahashi, Seward, Plotkin, Orenstein and Offit1]. Although varicella is usually self-limiting in children, infants aged <1 year, adults, and immunocompromised persons are at greater risk for severe complications that can require hospitalization or result in death [Reference Arvin2]. Although VZV infections occur worldwide, the epidemiology has been reported to differ between countries with tropical climates compared to those with temperate climates [Reference Lee3]. In temperate climates, varicella is a nearly universal disease of preschool and school-aged children with peak incidence occurring during late winter and early spring [Reference Wharton4]. Varicella seroprevalence studies from temperate countries, such as the USA [Reference Reynolds5] and western Europe [Reference Nardone6], report seropositivity rates of >90% in persons aged >15 years. However, in tropical countries, a greater proportion of adolescents and young adults have been found to be seronegative for antibodies to VZV [Reference Lee3]. As a result, these populations remain at risk for varicella at an older age, when it can result in higher rates of complications, hospitalizations, and deaths [Reference Gershon, Takahashi, Seward, Plotkin, Orenstein and Offit1].
The varicella immunization programme in the USA has resulted in a 90% reduction in varicella incidence in all age groups [Reference Guris7], a >65% decline in varicella-related hospitalizations [Reference Lopez8], and a 96% decline in varicella-related deaths in persons aged <50 years [Reference Marin, Zhang and Seward9]. American Samoa (AS), an unincorporated territory of the USA, plans to introduce varicella vaccination as part of their routine immunization programme although the specific timeline is unknown; varicella vaccine is not available in the public or private sector in AS at this time. With limited varicella surveillance data from the Pacific Islands, there is little known about the epidemiology of varicella in this region. We conducted a VZV seroprevalence study in AS of elementary and college students to provide baseline data to monitor the impact of a future vaccination programme on the epidemiology of varicella in AS.
METHODS
Study location
AS consists of six islands located between Hawaii and New Zealand in the South Pacific Ocean [10]. It lies at a latitude of 14° 20′ S and a longitude of 170° 00′ W. AS is a tropical island with an average temperature of 26·7°C, high humidity throughout the year, and average monthly rainfall of 251 mm. In 2010, the population of AS was 55519 with an estimated annual birth cohort of 1260 children; 95% of the population resides on the main island of Tutuila [11].
Study participants
The AS Department of Health in collaboration with AS Department of Education invited principals of all private and public elementary schools on the main island of Tutuila to participate in a seroprevalence study of vaccine-preventable diseases to assess immunity against hepatitis B, measles, mumps, rubella, and varicella in first-grade students. We also invited all students from the community college in AS, all of whom only attend college on the island, to participate in a study assessing varicella seroprevalence and measuring the anamnestic response to a single dose of hepatitis B vaccination.
This study was reviewed by the AS Department of Health Institutional Review Board and the CDC NCIRD Human Subjects Office. Written informed consent was obtained from participating college students and from parents of elementary students.
Study questionnaire, specimen collection and laboratory testing
A standard questionnaire, written in both English and Samoan, was distributed to parents/guardians of first-grade students of participating elementary schools and participating college students in order to collect demographic information, and self-reported varicella disease and vaccination histories. Varicella disease history was based on parental/guardian report for elementary school students, while this information was based on self-report for college students. Information on varicella vaccination history was also retrieved from immunization cards.
Five to ten millilitres of venous blood were collected during April–May 2011 from study participants. The blood samples were centrifuged and the sera was stored at −20°C at the main hospital in AS. Sera were transported on dry ice to the CDC National VZV Laboratory in Atlanta, GA and tested for VZV-specific immunoglobulin G (IgG) antibodies by using CDC's standard protocol. Specimens were tested by whole-cell enzyme-linked immunosorbent assay (ELISA); samples that yielded negative results were retested using the more sensitive IgG glycoprotein (gp) ELISA, as described previously [Reference Maple12, Reference Schmid and Jumaan13].
Analysis
Data were analysed using SAS version 9.3 (SAS Institute Inc., USA). We used Pearson's χ 2 or Fisher's exact tests to analyse categorical variables. A significant association was defined as one with a two-sided P value of <0·05. We calculated 95% exact binomial confidence intervals (CIs). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of self-reported history of varicella in comparison with VZV IgG results were calculated for elementary and college students separately.
RESULTS
Of the 36 elementary schools in Tutuila Island, students in 27 (75%) schools agreed to participate in the study. Adequate samples for VZV testing were collected from 39% (515/1310) elementary and 12% (208/1787) college students. The median age of participants was 6 years (range 4–9 years) for elementary school students and 20 years (range 17–35 years) for college students. There were 18 (3·5%) elementary students and one (0·5%) college student participant with one dose of varicella vaccination documented.
VZV seroprevalence increased from 76% in the 4–6 years group to 98% in those aged ⩾23 years. History of prior varicella disease for elementary school students was significantly associated with VZV seropositivity (Table 1).
Table 1. Seroprevalence of varicella in American Samoa for elementary and college students, 2011, by selected variables

CI, Confidence interval; n.a., not available.
* Excludes 16 students with missing information on gender.
† Excludes eight students with missing information on birth date or date of serum collection. No data were available on children aged 10–16 years.
‡ Excludes one student with missing information on region.
§ Place of birth was only available for 484 elementary school students.
∥ Eighteen students were born in the USA, one student was born in Marshall Islands, one student was born in Australia, and one student in Tonga.
A total of 651 study participants [comprising 89% (458/515) elementary school students and 93% (193/208) college students] provided information on varicella disease history (Table 2). The PPV and NPV of varicella disease history for VZV IgG positivity for elementary school students based on parental/guardian report was 93·4% (95% CI 89·1–96·3) and 36·4% (95% CI 30·4–42·8), respectively. The PPV and NPV of VZV IgG positivity for college students based on self-report was 97·6% (95% CI 93·3–99·5) and 3·0% (95% CI 0·4–10·5), respectively. For elementary school students, the sensitivity of reported disease history was 55·7% (95% CI 50·3–60·9) and specificity was 86·5% (95% CI 78·5–92·4); for college students, sensitivity was 66·0% (95% CI 58·7–72·7) and specificity was 40·0% (95% CI 5·3–85·3).
Table 2. Comparison of reported varicella disease history and VZV seropositivity status by reporting source, American Samoa, 2011
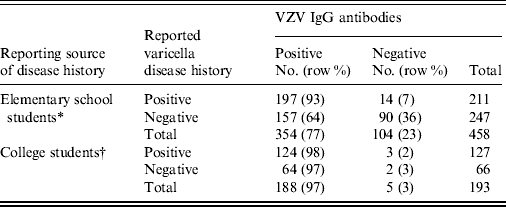
* Varicella disease history for elementary school students was based on report by parents or guardians of students.
† Varicella disease history for college students was based on self-report.
DISCUSSION
This is the first published report describing VZV seroprevalence in AS. Rates of VZV seropositivity were high for all age groups tested and increased substantially with age, with >97% of ⩾17-year-olds VZV seropositive. As in other studies of varicella seroprevalence conducted in populations prior to introduction of varicella vaccine, reported varicella disease history was highly predictive of VZV seropositivity for both elementary and college students in AS, whereas a negative history was a poor predictor of VZV seronegativity.
The high varicella seroprevalence rates we report for AS are similar to those found in recent studies from temperate countries [Reference Reynolds5, Reference Nardone6] and comparable to seroprevalence rates in the USA before the implementation of the one-dose routine varicella vaccination programme in 1995, although US seroprevalence was lower in children aged 4–10 years compared to this population [Reference Kilgore14]. The varicella seroepidemiology in AS reported in this study is higher than that in serosurveys conducted in other tropical island populations [Reference Lee3, Reference Bartoloni15–Reference Withers18]. A serosurvey conducted in young adult military recruits found that 38% from the Pacific Island nations of the Federated States of Micronesia and the Republic of the Marshall Islands during 1988–1990 were VZV seronegative, as were 42% from Puerto Rico during 1986–1987 [Reference Longfield17, Reference Withers18]. There are several possible explanations for differences in the seroepidemiology in AS compared to that previously reported for other islands and regions with similar climates. Daycare attendance by young children in AS has become quite common and high seroprevalence in AS could be facilitated by increased opportunities for transmission in young children attending daycare centres [Reference Wharton4, Reference Kudesia19]. Sera for some of the seroprevalence studies conducted in tropical climates were collected 20–30 years ago [Reference Garnett16–Reference Withers18] and may not be reflective of the current VZV epidemiology. Finally, results from our study may not be directly comparable to earlier studies that used less sensitive VZV serological assays [Reference Schmid and Jumaan13].
Because our study included participants from a limited number of birth cohorts, we cannot fully describe the epidemiology of varicella in AS. The high rates of VZV seropositivity in AS that we found may be related to endemic VZV transmission or the result of periodic epidemics of varicella which could result in nearly all susceptibles in this island population becoming infected. Although varicella is not a reportable condition in AS, Department of Health staff report that varicella appears to circulate continuously in this small island population and that there have been no large outbreaks of varicella observed in recent years.
We found that parental recall of prior varicella disease history was not a reliable indicator for VZV seroprevalence in AS, with only 46% of parents recalling history in elementary school students, yet a VZV seroprevalence rate of almost 80%. The discrepancy in parental report of varicella history and VZV seroprevalence that we found in our study may be due to off-island varicella vaccination history not captured by our study, the survey question translation into the local language, lower parental recognition of varicella in AS, or asymptomatic disease. Additional studies to evaluate the validity of parental recall in this population with laboratory testing may help to identify susceptible populations that should be vaccinated and prevent un-needed vaccinations. However, we did find a high PPV for reported history of varicella disease and VZV seropositivity for both elementary and college-aged students. These data suggest that report of prior varicella disease history in this population may be a reliable indicator of VZV seropositivity if a varicella vaccination programme is implemented in AS.
There were several limitations to this study. We used a convenience sampling methodology that was limited to selected age groups and information was not available for non-participants. However, given the relatively small population of AS, we believe that the VZV seroepidemiology we report is likely to be broadly reflective of the age groups tested. Because American Samoans can travel to and work freely in the USA, it is possible that some VZV-seropositive participants who reported no varicella disease history may have received doses of varicella vaccine while residing temporarily off-island that were not captured in AS immunization records. We were unable to collect information about place of birth from the college students and therefore it is possible that exposure to VZV for some students may have occurred outside AS.
The findings of our study demonstrate that there is high VZV seroprevalence in AS, even in the young, suggesting that the epidemiology of varicella in this tropical island country may be similar to that seen in more temperate climates. Varicella complications, although rare, have the potential to be fatal. In addition, varicella can result in lost days of school and work for affected families and require substantial public health resources to control outbreaks and manage severe disease [Reference Zhou20]. With a safe and highly effective vaccine available, introduction of a routine varicella vaccination programme can significantly reduce varicella disease burden as well as costs, resources, and time allocated for managing varicella cases and outbreaks [Reference Lopez8, Reference Marin, Zhang and Seward9, Reference Zhou20–Reference Rozenbaum22].
ACKNOWLEDGEMENTS
We thank Dr Jane Seward for her thoughtful review of the manuscript.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
T.D. was funded through the CDC Experience Applied Epidemiology Fellowship program, a public/private partnership supported by a grant to the CDC Foundation from External Medical Affairs, Pfizer Inc.






