Introduction
Although dairy products provide more bone-beneficial nutrients, such as protein, Ca, Mg, K, Zn and P per unit energy than any other typical food found in the adult diet( Reference Heaney 1 ), the relevance of dairy products for the prevention of osteoporotic fractures is still a matter of scientific debate as some large prospective studies have suggested that increased milk consumption during adolescence( Reference Feskanich, Bischoff-Ferrari and Frazier 2 ) or adult life( Reference Michaëlsson, Wolk and Langenskiöld 3 ) may be associated with a higher (future) hip fracture risk in men( Reference Feskanich, Bischoff-Ferrari and Frazier 2 ) or women( Reference Michaëlsson, Wolk and Langenskiöld 3 ). Yet, observational studies are only hypothesis generating and osteoporosis risk is influenced by multiple factors( Reference Heaney 4 , Reference Zengin, Prentice and Ward 5 ), including risk of falling( Reference Bonjour, Kraenzlin and Levasseur 6 ). There is broad consensus that high bone mineral density (BMD) will increase the likelihood of reduced osteoporosis and fracture risk in later life( Reference Weaver, Gordon and Janz 7 ). Therefore randomised controlled trials (RCT) should indicate efficacy of dairy products to promote BMD. Maximising bone mineral mass during childhood and adolescence, and thus achieving the highest possible peak bone mass at the end of the skeleton’s maturation process, may contribute to fracture risk reduction during adolescence and possibly in the elderly. Computer simulations of the bone remodelling process predict that the onset of osteoporosis will be delayed by 13 years( Reference Rizzoli, Bianchi and Garabédian 8 ) and the fracture risk during adult life will be reduced by 50 %( Reference Rizzoli 9 ), if peak bone mass is 10 % higher than the mean and sustained over time. In their review, Rizzoli et al. ( Reference Rizzoli, Bianchi and Garabédian 8 ) described that more than 60 % of the variance of peak bone mass is genetically determined, and that the remainder may be amenable to (lifestyle) interventions such as adequate dietary intake of Ca, protein and vitamin D, as well as regular weight-bearing physical activity( Reference Rizzoli, Bianchi and Garabédian 8 ).
With respect to dietary Ca intake, the findings of Esterle et al. ( Reference Esterle, Jehan and Sabatier 10 ) suggest that ethnic-specific polymorphisms of the vitamin D receptor gene promoter (VDRp) might play a role in the necessity of dairy product-derived Ca for lumbar spine bone mineral content (BMC) and BMD development, as shown during a 4-year follow-up of European peri- and post-menarcheal girls. On the other hand, research with monozygotic twins suggests that the relative importance of environmental factors for bone mass may increase with advancing age( Reference Slemenda, Christian and Williams 11 ).
Differences in fat mass, muscle strength, bone microarchitecture and/or physical activity levels play a role in bone mass( Reference Zengin, Prentice and Ward 5 ), since bone tissue adapts its internal structure and mass to mechanical demands to ensure maximal strength with minimal bone mass( Reference Frost 12 ). Humans literally walk on their Ca nutrient reserve; given an adequate Ca intake we have only as much bone as we need for the mechanical loads we currently experience( Reference Weaver and Heaney 13 ). Therefore, when trying to understand the role of dairy products for bone mineralisation and fracture risk during growth and ageing, other factors such as BMI, physical activity, baseline Ca intake and vitamin D status should be taken into account.
Given modern food sources, arguments have been put forward that it is difficult to devise a diet that is ‘bone healthy’ without including three servings of dairy products per d, as dairy foods are readily available sources of bone-relevant nutrients, and furthermore support skeletal muscle health( Reference Bonjour, Kraenzlin and Levasseur 6 , Reference Weaver, Gordon and Janz 7 , Reference Rizzoli, Abraham and Brandi 14 ). Many single studies underscore the beneficial or at least neutral effects of dairy products on bone. Nevertheless, the relevance of dairy produce for the diminishment of osteoporotic risk is still a matter of scientific debate. Systematic reviews and meta-analyses on RCT in the case of bone mineralisation or prospective studies in the case of fracture risk have been published regularly with a growing number of studies; the number of studies analysed differs per analysis, from a few studies only up to fourteen studies. Meta-analyses yield, besides a higher statistical power, a weighted average of available data, and therefore a more robust point estimate. This review will address the most up-to-date literature with the highest strength evidence possible on dairy products and bone health in a life-cycle perspective, with a primary focus on BMD/BMC and a reduction of fracture risk by including systematic reviews and meta-analyses. As the available systematic reviews and meta-analyses are heterogeneous in nature, we analysed in addition comparisons between dairy products and Ca supplements or other food products, vitamin D-fortified dairy and unfortified dairy products, as well as effects of different types of dairy products.
Methodology and scope
This review aims to present the most up-to-date literature with the highest strength evidence, i.e. meta-analyses and systematic reviews( 15 ) related to dairy products and BMC or BMD and fracture risk. The most optimal systematic reviews/meta-analyses included RCT on BMC or BMD and prospective studies on fracture risk. When other designs were included in the systematic review/meta-analyses, the discussion was restricted to RCT for BMD/BMC and prospective studies for fracture risk. The more recent individual RCT on BMD/BMC and prospective study on fracture risk, not yet included in the systematic reviews or meta-analyses, were separately discussed. To this end, we conducted an extensive literature search in Scopus and PubMed for papers published in the English language until January 2016, using combinations of the terms bone* OR fracture* OR BMD OR BMC OR osteoporosis OR osteopenia OR skelet*; dairy OR yoghurt OR yogurt OR milk OR cheese OR custard OR curd OR buttermilk OR kefir OR (“dietary calcium”); human OR humans OR people OR persons OR elderly OR children; AND supplement* OR food* OR (“dietary calcium”) OR vitamin* OR mineral* OR enrich* OR fortified OR fortify. Table 1 shows an overview of the preconceived in- and exclusion criteria. Main outcome measures of bone health were fractures for adults and children, BMD for adults, and BMC/BMD for children and adolescents. BMC is a better outcome measure of intake and bone mass in children and less dependent on bone size as opposed to BMD( Reference Huncharek, Muscat and Kupelnick 16 ).
Table 1 Overview of preconceived inclusion and exclusion criteria

DEXA, dual-energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography; RCT, randomised controlled trial.
Nutritional interventions that alter remodelling produce a temporary phase lag between the normally coupled resorption and formation of the bone-remodelling process. This is also called the bone remodelling transient described by Heaney( Reference Heaney 17 ), which is a temporary alteration in the balance between bone formation and bone resorption brought about by any factor that affects bone remodelling (for example, drugs, hormones or nutrients that alter either secretion of parathyroid hormone or its action on bone). According to Heaney( Reference Heaney 17 ), the optimal study length is just long enough to get a fix on the post-transient, steady state, which usually is a total duration of two to three remodelling periods, which according to their data begins at about 1·5 years in adults. The remodelling activity is spread out over several months (several weeks in growing children, approximately 3 months in young adults and 6–18 months in older adults( Reference Heaney 17 )). Although some think interventions with Ca or dairy product supplementation in children and adolescents are generally too short (1–3 years) to address the question of whether it is the temporary adaptation of bone tissue to the alteration in Ca intake that leads to peak bone mass( Reference Matkovic, Gael and Badenhop-Stevens 18 ), most RCT have been 1–2 years in duration( Reference Lanham-New 19 ). Because of this background and estimated annual losses of bone mass normally seen with ageing are in the range of about 1–2 % per year( Reference Lanham-New 19 ), we set a preferred minimal study duration for studies on BMD in adults of 2 years. Because of a shorter remodelling period in children, the preferred minimal study duration for studies on BMC in children was set at 1 year.
In total, six meta-analyses and two systematic reviews, describing thirty-three unique RCT and twenty-five prospective studies, and three individual prospective studies, were discussed that complied with our criteria. The available systematic reviews/meta-analyses are heterogeneous in nature and therefore we provide additional information that enabled us to review the outcome effects when comparing Ca- and/or vitamin D-fortified dairy products with non-fortified dairy products, Ca supplements or other food products. These studies included also single RCT and/or observational studies.
Dairy products and bone health in children and adolescents
Dairy products and bone mineral content/bone mineral density in children and adolescents
As shown in Table 2, our search for relevant papers on dairy products and BMD or BMC in children and adolescents yielded two meta-analyses on RCT and two systematic reviews on RCT and observational studies for discussion. In total, in these four papers, nineteen unique RCT were described of which fourteen showed a beneficial effect of dairy products/milk or Ca from dairy products/milk on BMC in children and four showed no effect.
Table 2 Meta-analyses (MA) and systematic reviews (SR) on dairy products and bone mineral content (BMC) or fracture risk in children and adolescents
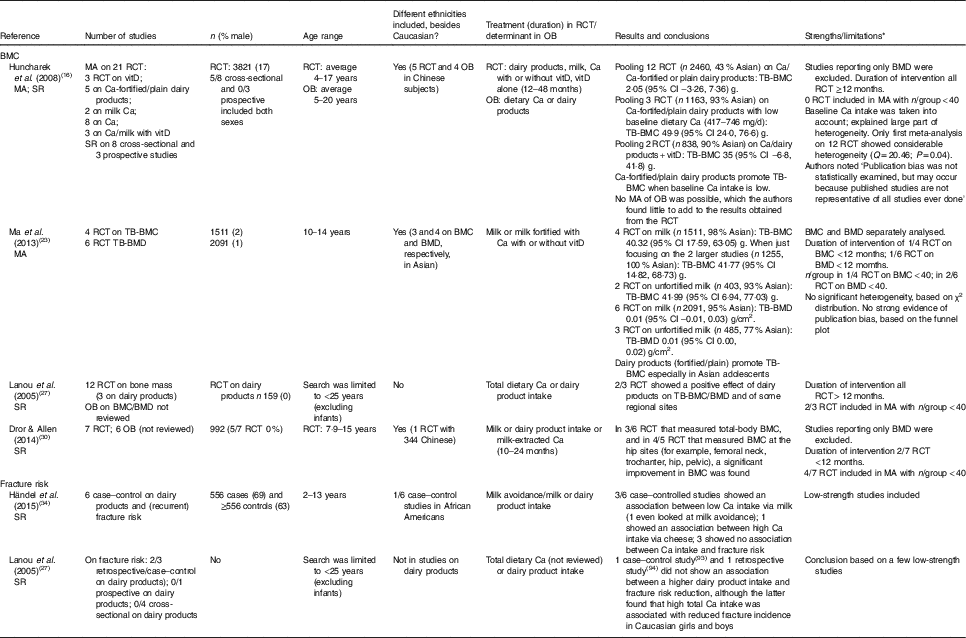
RCT, randomised controlled trial; OB, observational study; vitD, vitamin D; TB, total body; BMD, bone mineral density.
* BMC is better because it is less dependent on bone size as opposed to BMD. The minimum duration of intervention within RCT is of 12 months or longer. Based on most RCT an estimate of minimal n 40/group was set. Information given in each paper on heterogeneity or publication bias is literally copied, and when reported Q is given, this is a measure of the statistical significance of heterogeneity.
The earliest meta-analysis of Huncharek et al. ( Reference Huncharek, Muscat and Kupelnick 16 ) comprised twenty-one RCT that measured BMC. In total 2460 subjects were included in a pooled analysis, which showed no effect of dairy products (four studies) or Ca (eight studies) on total body BMC, with baseline Ca intake varying from 417 to 1198 mg/d. Pubertal status, age, study duration and site of BMC measurement varied widely across studies; however, sensitivity analyses suggested that baseline Ca intake explained most of the observed statistical heterogeneity. Subgroup analysis revealed that (fortified) dairy products significantly increased total body BMC by 50 (95 % CI 24, 77) g in children with low baseline Ca intakes (three RCT; n 1163; 417–746 mg/d). Only two of these studies used normal milk as the intervention (568 ml milk/d)( Reference Cadogan, Eastell and Jones 20 ). The other used milk fortified with Ca (245 mg/d) and with or without 3·3 µg vitamin D/d( Reference Du, Zhu and Trube 21 ) or milk powder with 600 or 1300 mg Ca/d( Reference Lau, Lynn and Chan 22 ).
The second meta-analysis( Reference Ma, Zheng and Ding 23 ) showed a comparable increase of 40 (95 % CI 18, 63) g in total body BMC (four RCT; n 1511) due to unfortified or Ca-fortified milk with/without vitamin D in children aged 10–14 years. Three of the four milk-based studies were not included in Huncharek’s meta-analysis( Reference Huncharek, Muscat and Kupelnick 16 ); one because of a small sample size( Reference Volek, Gómez and Scheett 24 ) and two Asian studies probably due to Chinese language in the source documents( Reference Tong and Zhang 25 , Reference Liu, Zhang and Ouyang 26 ). The beneficial effect of unfortified milk was in a similar range; however, one of these two studies had an intervention of only 3 months( Reference Volek, Gómez and Scheett 24 ).
The systematic review of Lanou et al. ( Reference Lanou, Berkow and Barnard 27 ) focuses on children and young adults (1–25 years) and reviews fifty-eight studies; only three RCT (n 159) studied dairy products in white girls who were aged 11–18 years and whose mean baseline Ca intakes ranged from 725 to 900 mg/d. One of these three RCT( Reference Cadogan, Eastell and Jones 20 ) was also part of the meta-analysis by Huncharek et al. ( Reference Huncharek, Muscat and Kupelnick 16 ), whereas the other two studies were not included in that review due to too low total study sample size (Matkovic et al. ( Reference Matkovic, Fontana and Tominac 28 ), n 31; Chan et al. ( Reference Chan, Hoffman and McMurry 29 ), n 48). Therefore, this review is not further discussed.
Recently Dror & Allen( Reference Dror and Allen 30 ) included in their systematic review six observational studies and seven RCT (duration 10–24 months; n 992) with healthy and well-nourished children or adolescents aged 2–19 years, using milk powder, Ca extracted from milk, or dairy products as the exposure and total-body or regional BMC as outcomes of interest( Reference Dror and Allen 30 ). The largest trial conducted in Asia( Reference Du, Zhu and Trube 21 ) was missing from this systematic review. We only will discuss the RCT. Five of the seven RCT were conducted in white female subjects. Baseline Ca intakes ranged from 417 to 900 mg/d. The treatments in the seven RCT did not contain vitamin D although one small RCT( Reference Chan, Hoffman and McMurry 29 ), conducted in the USA where mandatory fortification of milk with vitamin D is applied, might be an exception. Two studies investigated milk Ca( Reference Iuliano-Burns, Wang and Evans 31 , Reference Bonjour, Carrie and Ferrari 32 ). These three studies and the study of Merrilees et al. ( Reference Merrilees, Smart and Gilchrist 33 ) on dairy products were not included in the meta-analysis of Huncharek et al. ( Reference Huncharek, Muscat and Kupelnick 16 ). In all seven RCT there was a significant improvement in BMC due to higher dairy Ca intakes in at least one population subset at one or more bone sites; in three of the six RCT that measured total body BMC, and in four of the five RCT that measured BMC at the hip sites (for example, femoral neck, trochanter, hip, pelvic). Results at a particular site of measurement were inconsistent( Reference Dror and Allen 30 ).
In summary, as shown in Table 2, most studies were conducted in white female children and adolescents, and especially the larger studies were conducted in Chinese subjects with low basal Ca intake levels. These latter studies were included in the meta-analysis of Ma et al. ( Reference Ma, Zheng and Ding 23 ). The largest sample size was included in the meta-analysis of Huncharek et al. ( Reference Huncharek, Muscat and Kupelnick 16 ). Both meta-analyses underscore that plain dairy products or fortified with Ca and/or vitamin D improve total-body BMC when the daily baseline Ca intake is lower than 750 mg. The systematic review of Dror & Allen( Reference Dror and Allen 30 ) confirmed this for 50 % of their selected RCT on total-body BMC. The role of dairy products is less clear for the regional sites of BMC.
Dairy products and fracture risk in children and adolescents
With regard to the relevance of dairy products in reducing fracture risk in childhood, only observational data of lower scientific strength are available. Two systematic reviews( Reference Lanou, Berkow and Barnard 27 , Reference Händel, Heitmann and Abrahamsen 34 ) were not analysed in detail as these reviews contained mostly case–control studies (see Table 2). However, one prospective study was not discussed in these systematic reviews. This cohort study with 22 years of follow-up in more than 96 000 white postmenopausal women and men aged 50 years and older suggested that high consumption of milk during adolescence may adversely influence future fracture risk by increasing height for at least 22 years( Reference Feskanich, Bischoff-Ferrari and Frazier 2 ). Frequency of consumption of milk during teenage years and attained height during ages 13 to 18 years were reported at baseline, which was by surprise at an age over 40 years. Among those who consumed at least four glasses of milk per d, men were on average 1·9 cm taller and women were 1·7 cm taller than those who consumed fewer than two glasses per week during teenage years. Each additional glass of milk per d during teenage years was associated with a significant 9 % higher risk of hip fracture in men, but not in women. The positive association observed in men was partially mediated through attained height, as was expected by the authors because of presumed vulnerability to bone fracture due to increased height associated with milk exposure. Unlike milk, cheese consumption during teenage years was not associated with hip fractures in men or women; neither was it associated with attained height. Because of method limitations (for example, not including many important confounders or including too many like current Ca intake) in their study design and data analysis, the conclusions of Feskanich et al. ( Reference Feskanich, Bischoff-Ferrari and Frazier 2 ) were not considered valid by Howland( Reference Howland 35 ), Weaver( Reference Weaver 36 ) or Heaney( Reference Heaney 37 ). In their reply Feskanich & Willett( Reference Feskanich and Willett 38 ) stated that other confounders were not of importance because they were not associated with the determinant/outcome and that excluding the current Ca intake did not change their outcome. Besides the arguments forwarded in these three publications, another shortcoming is the one-time assessment of dairy product intake during teenage years, assessed at a later age by a short twenty-three-item FFQ. A one-time assessment of dairy product intake does not predict intake over very long follow-ups, as was shown in the Amsterdam Growth and Health Study in which Ca and dairy product intake was studied six times over 15 years from age 13 to 27 years. The predictability of Ca intake over time was not sufficiently strong to identify teenagers who are likely to maintain an inadequate Ca intake in adulthood( Reference Welten, Kemper and Post 39 ). Therefore, predicting fracture risk based on adolescence dietary behaviour seems rather difficult, if not impossible.
Conclusions on dairy products and bone health for children and adolescents
In conclusion, especially based on two meta-analyses( Reference Huncharek, Muscat and Kupelnick 16 , Reference Ma, Zheng and Ding 23 ), plain dairy products or fortified with Ca and/or vitamin D improve total-body BMC of Chinese and Caucasian girls by 45–50 g over 1 year when the daily baseline Ca intake is lower than 750 mg. This is comparable with the higher total BMC of 117 g over 2 years surrounding the peak in bone accretion found in boys and girls, when being physically active( Reference Bailey, McKay and Mirwald 40 ). No conclusion can be drawn on the regional sites of BMC. Substitution of a deficiency appears more effective than augmentation above a normal intake.
With regard to childhood fracture risk, no conclusion can be drawn based on the two systematic reviews because of the low strength of scientific evidence of the described studies. Further study is also needed on the association between multiple assessments of dairy product intake during childhood for future fracture risk in adult life, although the necessary very long follow-up of at least 20–30 years makes this almost impossible to execute. Also, an enormous number of subjects would need to be included to be able to face the drop-outs and still have enough power to see differences in fracture risk after a follow-up of more than 20 years.
Dairy products and bone health in adults
Dairy products and bone mineral density in adults
One systematic review and one meta-analysis on RCT were found with regard to the relevance of dairy products for BMD in adults (see Table 3). The systematic review of Weinsier & Krumdieck( Reference Weinsier and Krumdieck 41 ) reported seven RCT, along with a number of cross-sectional and case–control studies. Of these RCT, four were conducted in children and adolescents and only three in adult or postmenopausal women (two included in the meta-analysis mentioned below and one prospective study erroneously classified as RCT). Therefore, we will not include the outcome of this review in our conclusion on adult bone health.
Table 3 Meta-analyses (MA) or systematic reviews (SR) on the effect of dairy products on bone mineral density (BMD) or fracture risk in adults

RCT, randomised controlled trial; OB, observational study; vitD, vitamin D; TB, total body; LS, lumbar spine; FN, femoral neck; FU, follow-up period; RR, relative risk.
* The minimum duration of intervention within RCT is 24 months or longer. Based on most RCT an estimate of minimal n 40/group was set. Information given in each paper on heterogeneity or publication bias is literally copied and when reported the measures are given (Q is a measure of the statistical significance of heterogeneity, and the I 2 index is a measure of the extent of heterogeneity).
Recently, a random-effects meta-analysis of fifteen RCT was performed to determine whether increasing Ca intake from dietary sources affects BMD, of which three were on hydroxyapatite( Reference Tai, Leung and Grey 42 ). We restrict to the seven RCT that intervened for a duration of 2 years; i.e. six RCT on dairy products (n 805) and one on hydroxyapatite (n 60). Between 800 and 1200 mg of Ca by dairy products and 3320 mg Ca by hydroxyapatite were supplied per d. One RCT included male participants, and one of the seven RCT supplied 20 µg vitamin D/d. Based on the title of the paper, about 46 % of the total numbers of participants included in the seven RCT on dietary Ca were part of the Chinese population (n 400). More details are given in Table 3. The meta-analysis showed that a higher Ca intake from dietary sources increased BMD by 0·7–1·8 % at the lumbar spine, total body, total hip and femoral neck after 2 years. The number of subjects per site of BMD measurement was between 358 and 816, and P values were 0·001 or lower. A beneficial effect on BMD of the forearm could not be demonstrated (0·1 %; P=0·65; n 171). The authors mentioned that there is a possibility of publication bias due to the presence of more small- to moderate-sized highly positive studies, indicating that larger studies are needed. When excluding the trials on hydroxyapatite, the authors mentioned there was little change in the results.
A subgroup analysis showed no significant difference in change in lumbar spine BMD when baseline dietary Ca intake was above or below 800 mg/d (P=0·54; four and five RCT, respectively). Unfortunately, this analysis was only feasible for the lumbar spine measurements, comparing different studies, at 1 year of intervention.
Based on one meta-analysis, a 2-year intervention with a higher Ca intake, mainly from dairy products, increased BMD of the lumbar spine, total body, total hip and femoral neck by 0·7–1·8 % in participants aged 50 years or older at baseline.
Dairy products and fracture risk in adults
The role of dairy products with respect to fracture risk was investigated in three meta-analyses, which included in total fourteen unique prospective studies. Of these, twelve prospectively found a beneficial association between the intake of milk, dairy protein, a dietary pattern including dairy products, or dietary Ca, and fracture risk at one or more sites. Besides the meta-analyses we reviewed one systematic review of prospective studies, and two recent prospective studies, not included in the previous analyses (see Table 3).
Xu et al. ( Reference Xu, McElduff and D’Este 43 ) included in their meta-analysis three study designs to determine whether a low dietary Ca intake from different types of foods is one of the risk factors for hip, forearm or vertebral fractures in women aged >35 years. They selected four prospective studies (n 39 780 residents from USA and Europe) with a mean follow-up period between 5·2 and 14·6 years. Only one meta-analysis could be done: no significant association was found between dietary Ca intake and hip fractures (relative risk (RR) 0·96; 95 % CI 0·89, 1·04). The limitation of this meta-analysis is that no details on dietary Ca sources were provided, although 73 % of the subjects originated from prospective studies aimed to study the role of dairy products in fracture risk. Although included in the meta-analysis of Bischoff-Ferrari et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ), the second meta-analysis of Kanis et al. ( Reference Kanis, Johansson and Oden 45 ) is unique in pooling individual data from 39 563 adults and elderly from six prospective studies carried out in Europe, Australia and Canada. These data were not included in the meta-analysis of Xu et al. ( Reference Xu, McElduff and D’Este 43 ). This meta-analysis of individual patient data assessed whether low Ca intake, estimated from the intake of milk (but not cheese, yoghurt or supplements), could be used to serve as an additional risk factor for case finding of osteoporotic patients. The incidence of any, osteoporotic or hip fracture over 3–8 years was 3191, 2469 and 413, respectively. When adjusted for BMD, which could be considered to be an effect modifier and therefore as expected, a low intake of milk was not associated with fracture risk (any, hip or osteoporotic) for the various age categories analysed above 50 years of age. There was no difference in risk ratio between men and women. When not adjusted for BMD, the risk ratio for having an osteoporotic fracture with low milk intake was borderline significant (RR 1·10; 95 % CI 1·00, 1·21; P=0·056). When RR was examined by age in men and women combined, drinking less than one glass of milk per d was associated with an increased risk of osteoporotic fracture only from the age of 80 years (RR 1·15; 95 % CI 1·02, 1·30). This was not the case for hip fracture or any fracture( Reference Kanis, Johansson and Oden 45 ).
The third meta-analysis of Bischoff-Ferrari et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ) focused solely on hip fractures in middle-aged or older men and women, and included the data from Kanis et al. ( Reference Kanis, Johansson and Oden 45 ). Compared with the meta-analysis of Xu et al. ( Reference Xu, McElduff and D’Este 43 ), Bischoff-Ferrari et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ) included only studies that gave information on milk intake; therefore there is only overlap of two studies( Reference Cumming, Cummings and Nevitt 46 , Reference Meyer, Pedersen and Løken 47 ). Bischoff-Ferrari et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ) did not find an association between milk intake and hip fracture risk in women (195 102 women, 3574 hip fractures, pooled RR per glass of milk (approximately 300 mg of Ca) per d=0·99; 95 % CI 0·96, 1·02). The authors mentioned that the results among women were somewhat dominated by the study of Michaëlsson et al. ( Reference Michaëlsson, Melhus and Bellocco 48 ) with 60 689 participants, although the test for heterogeneity did not reach significance when including all studies. Excluding the study of Michaëlsson et al. ( Reference Michaëlsson, Melhus and Bellocco 48 ), there was a marginally significant 5 % reduction of hip fracture risk per glass of milk intake per d (pooled RR 0·95, 95 % CI 0·90, 1·00; P=0·049). Moreover, the proportion of total variation in study estimates, due to heterogeneity, was 25 % lower when excluding the Swedish study by Michaëlsson et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ). More data are needed in men (75 149 men, 195 hip fractures) for whom the pooled RR per daily glass of milk was 0·91 (95 % CI 0·81, 1·01), suggesting a borderline significant benefit in men. When cohorts of both women and men were analysed jointly, there was a weak and non-significant inverse association between milk intake and hip fracture risk (pooled RR per glass of milk per d=0·97, 95 % CI 0·93, 1·01). All three meta-analyses included data from countries where mandatory fortification of milk with vitamin D is applied.
Bolland et al. ( Reference Bolland, Leung and Tai 49 ) recently published a systematic review of randomised controlled and prospective cohort studies on Ca intake with fracture as an endpoint. Regarding Ca from dairy products or milk, they found one RCT with milk powder( Reference Lau, Woo and Lam 50 ) (n 200; one fracture in the milk group and three in the controls in 2 years’ time; P=0·34), and eighteen prospective studies with sample sizes varying between 257 and 75 879 subjects. Six of the eighteen included cohort studies consisting of 100 % women. For nearly all eighteen included prospective studies on milk and/or dairy product intake (both vitamin D-fortified and -unfortified milk) there was no association with fracture risk (total, vertebra, forearm, hip), with twenty-five of twenty-eight neutral associations for milk and eleven of thirteen for dairy product intake( Reference Bolland, Leung and Tai 49 ). As compared with the two other meta-analyses, a larger number of studies were included in the systematic review of Bolland et al. ( Reference Bolland, Leung and Tai 49 ) because of a different aim (for example, milk v. Ca intake, or type of fracture); the prospective study of Munger et al. ( Reference Munger, Cerhan and Chiu 51 ) was probably excluded from the meta-analysis because it focused on protein intake. Bolland et al. ( Reference Bolland, Leung and Tai 49 ) concluded that dietary Ca intake is not associated with risk of fracture, and that there is currently no evidence that increasing dietary Ca intake to at least 1000–1200 mg/d prevents fractures. The limitation of this systematic review is that there was no meta-analysis performed to obtain a pooled and weighted average from the results of the individual studies on dietary or dairy Ca.
Finally, we discuss two large prospective studies that were not included in the described papers before. Since milk products in Australia and Sweden are not mandatorily fortified with vitamin D, and vitamin D intake was included as a confounder, we assume that the associations mentioned are related to unfortified dairy products. Khan et al. ( Reference Khan, Nowson and Daly 52 ) followed up for about 12 years on a total of 41 514 Australian men and women, aged 40–69 years at baseline. Overall there were about 10 % incident fractures. A higher dietary Ca intake (adjusted for Ca intake from supplements) was associated with a lower fracture rate (hazard ratio (HR) 0·70; 95 % CI 0·54, 0·92; P<0·004). Because milk products and dishes are the major source of Ca in the Australian diet (providing 42 % of the dietary intake of Ca), secondary analyses revealed that, compared with the reference quartile (<18 dairy servings/week), a higher dairy product intake (quartile 4; >48 servings/week) was associated with a decreased risk of fractures after 50 years of age (OR 0·78; 95 % CI 0·62, 0·99).
A second large prospective study published by Michaëlsson et al. ( Reference Michaëlsson, Wolk and Langenskiöld 3 ) drew much attention. The mean Ca intake of women in this study was between 733 mg/d (mean 60 g milk/d; 37·1 % Ca supplement users) and 1101 mg/d (mean 680 g milk/d; 23·2 % Ca supplement users), whereas the study of Khan et al. ( Reference Khan, Nowson and Daly 52 ) reported that the respective 5th and 95th percentiles of dietary Ca intake for women were 383 and 1555 mg/d. For women who consumed three or more glasses of milk per d (mean 680 g) as compared with less than one glass per d (mean 60 g), Michaëlsson et al. ( Reference Michaëlsson, Wolk and Langenskiöld 3 ) found an HR for any fracture of 1·16 (95 % CI 1·08, 1·25) and for hip fracture of 1·60 (95 % CI 1·39, 1·84). For every glass of milk in women no reduction in risk was observed with higher milk consumption for any fracture (HR 1·02; 95 % CI 1·00, 1·04) or for hip fracture (HR 1·09; 95 % CI 1·05, 1·13); the corresponding adjusted HR in men were 1·01 (95 % CI 0·99, 1·03) and 1·03 (95 % CI 0·99, 1·07). This negative effect was hypothetically explained by an effect of galactose, which in animal models may accelerate senescence due to an increase in oxidative stress and inflammation( Reference Michaëlsson, Wolk and Melhus 53 ). The study by Michaëlsson et al. ( Reference Michaëlsson, Wolk and Langenskiöld 3 ) used data from two large Swedish cohorts, one with 61 433 women (39–74 years at baseline 1987–1990) and one with 45 339 men (45–79 years at baseline 1997). The average follow-up was 20·1 years for women and 11·2 years for men( Reference Michaëlsson, Wolk and Langenskiöld 3 ). The study included a larger number of subjects than the total number included in the meta-analyses of previous studies. Still this sample size and neither the sample sizes included in the meta-analyses reviewed in this paper might not be high enough according to Kanis et al.’s estimations( Reference Kanis, Johansson and Oden 45 ), which are based on a correlation between milk intake and BMD as outcome, and predict that in order to establish a significant association with fracture risk a sample size of at least 500 000 subjects would be required.
Conclusions on dairy products and bone health for adults
In conclusion, based on one meta-analysis( Reference Tai, Leung and Grey 42 ) in primarily Caucasian women, increasing Ca intake from dairy sources with or without vitamin D increased BMD by 0·7 to 1·8 % at the lumbar spine, total body, total hip or femoral neck at 2 years. This increase in BMD is less than one-fifth of the increase in BMD after pharmaceutical intervention( Reference Tai, Leung and Grey 42 ). However, as compared with the loss of BMD of about 1 to 2 % during ageing( Reference Lanham-New 19 ), it seems clinically relevant. For lumbar spine BMD, based on a few comparisons the increase seems to be independent of baseline dietary Ca intake. However, this could not be investigated for the other sites of BMD and therefore needs further study.
On the contrary, based on four systematic reviews( Reference Bolland, Leung and Tai 49 )/meta-analyses( Reference Xu, McElduff and D’Este 43 – Reference Kanis, Johansson and Oden 45 ) and two prospective studies( Reference Michaëlsson, Wolk and Langenskiöld 3 , Reference Khan, Nowson and Daly 52 ) with contrasting results, dairy products, either or not fortified with vitamin D, do not play a role or only to a small extent of about 5 % in the reduction of hip fracture risk in a Caucasian population of mostly women. Based on pooled individual patient data, there are some indications that at very high age, osteoporotic fracture risk decreases with a higher milk intake.
Dairy products v. calcium supplements
For adults, one systematic review and meta-analysis is discussed that included RCT on BMD( Reference Tai, Leung and Grey 42 ). Using a random-effects model, two subgroups including the effects of dietary Ca sources v. Ca supplements were compared by a test for interaction between subgroups. For this purpose, seven studies on dietary sources of Ca, of which six were on dairy products, were compared with thirty-four studies on Ca supplements. Three RCT overlap since they studied both forms of Ca. The Ca dose of the trials on dietary sources and supplements was in 86 and 68 % of cases≥1000 mg/d, and in 0 and 14 %≤500 mg/d, respectively. In all trials on dietary sources of Ca, community-living participants were included, while in the trials on Ca supplements also participants living in institutions were included (12 %). Baseline Ca intake was≤800 mg/d in 86 and 52 % of the RCT on dietary sources or supplements, respectively. After 2 years of intervention, there was no difference in change of BMD at the lumbar spine, total hip and total body (0·7–1·8 % v. 0·8–1·5 %), except for BMD at the femoral neck and forearm. In the case of femoral neck BMD, this was in advantage of dietary sources of Ca, i.e. 1·8 (95 % CI 1·1, 2·6) v. 1·0 (95 % CI 0·5, 1·4) % (P interaction=0·05). While for BMD at the forearm, the change was bigger due to the supplement intervention (1·5 v. 0·1 %; P interaction=0·01). A closer look revealed that for the comparison of changes in BMD at the forearm and total body, only two RCT on dietary sources compared more than six RCT on supplements. For the other sites of BMD, a sufficiently high number of RCT of more than five trials on both forms were compared( Reference Tai, Leung and Grey 42 ).
Since different studies were compared with different baseline Ca and dosages, the authors also did a pooled analyses of multi-arm trials permitting a direct comparison of a Ca supplement arm with a dietary source of Ca arm. Out of seven multi-arm RCT, three trials (n 268) directly compared a dietary source of Ca arm (two comprising dairy sources) and a Ca supplement arm over 2 years’ time. Baseline Ca intake was about 800 mg/d and comparable for the two different Ca interventions. In the trial on hydroxyapatite the dose of Ca was lower for the dietary source (2500 mg/d) than the supplement (3320 mg/d), while in the dairy product intervention trials 1000 mg Ca/d in both interventions was given. Only for two bone sites, more than one RCT was included. For the lumbar spine three RCT (two comprising dairy sources) comparing dairy Ca/hydroxyapatite complex with supplements (not specified), the pooled analysis showed a non-significant BMD difference of –0·3 (95 % CI –1·3, 0·6) % (P=0·46). For the femoral neck, the pooled analysis also failed to show a significant BMD difference between dairy Ca and supplements (two RCT; 0·7 (95 % CI –1·3, 2·8) %; P=0·47). According to these authors the similar effect due to increased intake of Ca through dietary sources or supplements suggests that the non-Ca components of the dietary sources of Ca do not directly affect BMD( Reference Tai, Leung and Grey 42 ).
In conclusion, based on one meta-analysis( Reference Tai, Leung and Grey 42 ) only when comparing different studies with a different background on baseline Ca intake, the femoral neck may seem to profit more from dietary sources than from supplements. Based on the meta-analysis of two to three RCT in adult participants that directly compared the efficacy of dairy products with Ca supplements; dairy products are not better for BMD than Ca supplements.
Vitamin D-fortified v. -unfortified dairy products
There is one meta-analysis on RCT that compared fortified with non-fortified dairy products on bone health. A subgroup analysis in the meta-analysis of Tai et al. ( Reference Tai, Leung and Grey 42 ) showed no difference in effect of Ca monotherapy via dietary sources (eight RCT: BMD change 0·5 (95 % CI –0·4, 1·5) %) v. co-administered vitamin D (three RCT: BMD change 0·8 (95 % CI 0·2, 1·4) %) on BMD at the lumber spine after 1 year (P=0·62). For the other BMD sites or a longer intervention duration no comparison was possible. The daily dosages ranged between 300 and 800 IU (15 and 20μg) vitamin D. The average baseline 25-hydroxyvitamin D (25(OH)D) levels was in all included RCT higher than 50 nmol/l, thus sufficient, and therefore no additional effect of extra vitamin D on bone can be expected. More studies, especially in participants with vitamin D insufficiency (25(OH)D<50 nmol/l), are needed to conclude whether vitamin D-fortified dairy products are better for bone mass than unfortified dairy products.
Types of dairy products
Most studies analysed were on milk. In prospective cohorts, the beneficial effects on BMD or hip fracture risk found for high v. low dairy product intake seem to be mediated mainly by milk and yogurt( Reference Sahni, Tucker and Kiel 54 , Reference Sahni, Mangano and Tucker 55 ), which is probably related to their level of consumption. The definition of dairy product estimates consumed may differ in individual studies. The nutrient concentration on a weight basis is greater in yogurt and cheese than in milk, but serving sizes are typically less for these products than for milk( Reference Weaver 56 ). We did not find RCT that compared comparable intakes of Ca and protein from different types of dairy products, which might be due to the fact that blinding of treatments is impossible. Since meta-analyses and individual RCT are lacking comparing different types of dairy products, we included prospective studies. Sahni et al. ( Reference Sahni, Tucker and Kiel 54 , Reference Sahni, Mangano and Tucker 55 ) compared milk with other products made from milk in relation to bone health. In one study( Reference Sahni, Tucker and Kiel 54 ) Sahni et al. followed 3724 adults aged 55 years (range 26–85 years) for 12 years. Exposure variables were quartiles or tertiles of milk, cheese, yogurt, cream (cream+sour cream+ice cream+cream cheese), fluid dairy product intake (milk+yogurt drinks), and total dairy product intake. In final models, simultaneously including dairy foods and therefore adjusting for each other, yogurt intake remained positively associated with trochanteric BMD (P=0·04), while cream intake tended to be negatively associated with femoral neck BMD (P=0·08). Milk and yogurt intake showed a positive but non-significant trend with femoral neck BMD (P=0·06 and 0·09, respectively). No associations were observed for cheese intake or for lumbar spine BMD( Reference Sahni, Tucker and Kiel 54 ). These data were extracted from the Framingham Offspring Study, which was performed in the USA and therefore includes mandatory fortification of milk with vitamin D. The same research group did a similar kind of study in older subjects (n 803)( Reference Sahni, Mangano and Tucker 55 ). The average age was 77 years (range 68–96 years). A total of ninety-seven hip fractures occurred over the mean follow-up time of 11·6 years. Participants with a medium milk intake (>1 and <7 servings/week) or higher milk intake (≥7 servings/week) tended to have lower hip fracture risk than those with low (≤1 serving/week) intake (HR high v. low intake 0·58; 95 % CI 0·31, 1·06; P=0·078; and HR medium v. low intake: 0·61; 95 % CI 0·36, 1·08; P=0·071). There appeared to be a threshold for milk, with a 40 % lower risk of hip fracture among those with medium or high milk intake (7 servings/week) compared with those with low intake (P=0·061). Similarly, participants with a medium/high milk+yogurt intake (>1 serving/week) appeared to have a20 % lower risk of hip fracture compared with those with low milk+yogurt intake (<1 serving/week; P=0·104). These associations were further attenuated after adjustment for femoral neck BMD. No significant associations were seen for other dairy foods (P range=0·117–0·746). Taken together, these results suggest that greater intakes of milk and milk+yogurt may lower risk for hip fracture in older adults through mechanisms that are partially, but not entirely, attributable to effects on BMD( Reference Sahni, Mangano and Tucker 55 ).
Dairy products v. other foods
To be able to answer the question how dairy products compare with other foods in relation to bone health, we broadened our search strategy and included studies that address specific questions on the use of different protein or phosphate sources, or whether dairy product-free diets are appropriate enough to build strong bones. The systematic review and meta-analysis of observational studies in children at the ages of 2–13 years, primarily case–control in design (sixteen of eighteen), described by Händel et al. ( Reference Händel, Heitmann and Abrahamsen 34 ), showed a higher upper and lower limb fracture risk with milk avoidance (based on six case–control studies), or with a higher intake of sugar-sweetened carbonated beverages (three studies)( Reference Händel, Heitmann and Abrahamsen 34 ). The latter is probably a ‘replacement effect’, as suggested by the data of Whiting( Reference Whiting, Healey and Psiuk 57 ), showing that milk beverage intake in both boys and girls was inversely related to low nutrient-dense beverage consumption. In addition, girls, but not boys, have reduced bone mineral accrual when low nutrient-dense beverages replace milk beverages( Reference Whiting, Healey and Psiuk 57 ).
Recently, 410 children aged 9–12 years were randomly assigned to one of six groups to receive 200 ml of unfortified milk, fortified milk, unfortified orange juice, or fortified orange juice daily, or a supplement or placebo for 12 weeks. Plain milk supplied 240 mg Ca/d and plain orange juice 180 mg Ca/d. Both fortified milk and fortified juice supplied 2·5 µg vitamin D and 500 mg Ca/d. No significant difference was found in the changes of serum osteocalcin over time between plain milk and plain orange juice (27 v. 13 µg/l) or vitamin D- and Ca-fortified products (30 v. 24 µg/l)( Reference Neyestani, Hajifaraji and Omidvar 58 ). Osteocalcin serves as a bone formation marker, although serum carboxylated osteocalcin concentrations and, more strongly, the ratio of carboxylated:total osteocalcin, predict the occurrence of fractures in older community-dwelling adults much better than total osteocalcin( Reference Luukinen, Käkönen and Pettersson 59 ).
With regard to adults, one longer-term study on femoral neck and hip BMD (1·5 years) in Chinese postmenopausal women showed a higher impact of milk v. soya drinks. Both drinks supplied 250 mg Ca daily. No effect was seen on the lumbar spine( Reference Gui, Brašić and Liu 60 ). Various comparisons such as meat v. milk (same protein levels) or cola beverage v. milk or different P sources, among which meat and cheese (same P level; different Ca level) indicate a more beneficial effect of dairy products on markers of bone turnover and resorption in young adults( Reference Budek, Hoppe and Michaelsen 61 – Reference Kristensen, Jensen and Kudsk 63 ). These short-term dietary effects (24 h up to 10 d diet) of course need confirmation in long-term intervention studies with BMD as the outcome.
There are insufficient studies which mostly measure bone markers instead of BMD or BMC that allow for a good comparison between the effect of dairy products and other foods on bone health. The available data seem to indicate that dairy products may be more beneficial for building/maintaining bone mass than sugar-sweetened carbonated beverages or Ca-fortified soya drinks, which is probably related to the number of bone-relevant nutrients in the dairy package. There are gaps in our knowledge, however. We feel that some priority should be given to the (potential) replacement effect by sugar-sweetened carbonated beverages in children.
Discussion
The role of dairy products either with or without vitamin D, for total-body BMC of Chinese and Caucasian children/adolescents with a low Ca intake, and for BMD of the total body, lumbar spine, total hip and femoral neck in mainly Chinese and Caucasian adult women has been sufficiently established. Recently, a review of the global Ca map revealed that there are many countries where Ca intake is very low, under 600 mg/d( Reference Balk, Adam and Langberg 64 ). Especially countries in Asia-Pacific region, South America and scattered throughout the Far East, and North Africa may be more likely to benefit from additional dairy products.
While no studies have been done on the association between dairy products and fracture risk in children/adolescents, many studies have been done on (older) adults. Tai et al. ( Reference Tai, Leung and Grey 42 ) predicted a 1–2 % increase in BMD to produce a 5–10 % reduction in risk of fracture in adults, which is more than five times lower than observed with weak anti-resorptive agents. Therefore, a large sample size of about four to five times the sample sizes used in the meta-analyses or prospective studies described is needed to be able to study this relationship properly. There are two large Swedish studies on high intakes of milk (more than 600 ml/d) and fracture risk. The oldest one showed a neutral association, while the more recent one showed a detrimental association in women but not men( Reference Michaëlsson, Wolk and Langenskiöld 3 , Reference Michaëlsson, Melhus and Bellocco 48 ). Given the northern latitude and small amount of vitamin D fortification in fat-reduced milk in Sweden, decreased vitamin D exposure may explain in part the influence of the Swedish study toward a neutral association between milk intake and hip fracture risk, as also pointed out by Bischoff-Ferrari et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ). In 116 women included in the cohort of the first executed Swedish study( Reference Michaëlsson, Melhus and Bellocco 48 ), about 20 % had 25(OH)D concentrations of less than 50 nmol/l during the winter of 2006( Reference Burgaz, Åkesson and Annette 65 ). In addition the level of milk consumption is much higher than the mean intake of adults in most countries, and it is worth noting that dietary questionnaires were performed in 1987–1990 and 1997, when milk in Sweden was fortified with a high dose of vitamin A; high levels of vitamin A have been linked to increased risk of fracture( Reference Rozenberg, Body and Bruyère 66 , Reference Michaëlsson, Lithell and Vessby 67 ). Although both studies adjusted for retinol intake by including it as a confounder, the question is whether this is valid. Vitamin A has a high variance ratio (ratio of intrasubject to intersubject variances) and therefore assessment of intake requires many days of dietary data( Reference Whiting and Lemke 68 ) and it is unclear to what extent this has been validated in the Swedish cohorts. In addition, older adults as included in both studies lose the capacity to clear high levels of ingested retinol( Reference Whiting and Lemke 68 ) and therefore intake might not reflect status. When leaving out one of the Swedish studies( Reference Michaëlsson, Melhus and Bellocco 48 ) from a large well-controlled meta-analysis( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ), a marginally significant reduction of hip fracture risk of 5 % per glass of milk/d was found in women. This was recently confirmed in a very large individual prospective study( Reference Feskanich, Meyer and Fung 69 ), especially set up to compare with the results from the Swedish study( Reference Michaëlsson, Wolk and Langenskiöld 3 ) and to address the relative lack of data in men. In this cohort 80 600 women and 43 306 men over 50 years of age were followed for up to 32 years. Per daily serving of milk, that was assessed every 4 years, a significant 8 % lower risk of hip fracture was found in men and women combined (RR 0·92; 95 % CI 0·87, 0·97)( Reference Feskanich, Meyer and Fung 69 ). In addition, based on an individual patient data cohort, especially at an age older than 80 years, milk intake can reduce the risk on osteoporotic fractures (5–15 %). These percentages reductions confirm the estimations of Tai et al. ( Reference Tai, Leung and Grey 42 ).
Vitamin D status is a well-known factor, important for bone health. Only the meta-analysis of Tai et al. ( Reference Tai, Leung and Grey 42 ) compared vitamin D-fortified with unfortified dairy products. However, based on small short-term RCT they could not establish a higher BMD in the case of vitamin D fortification. All over the world, an adequate supply of both Ca and vitamin D is part of the nutritional recommendations to keep bone healthy. Appropriate levels of both dietary Ca and sufficient serum vitamin D levels are important for skeletal health( Reference Carmeliet, Dermauw and Bouillon 70 ), which is confirmed by several meta-analyses( Reference Avenell, Mak and O’Connell 71 , Reference Lips, Gielen and van Schoor 72 ). However, by using standardised serum 25(OH)D data, vitamin D insufficiency (25(OH)D<50 nmol/l) in Europe and North America can be classified as a severe (>40 %) public health problem. Fortification of a wider range of foods is likely to have the potential to increase vitamin D intakes. For both a vitamin D-fortified, reduced-fat cheese and vitamin D-biofortified eggs a high efficacy has been shown( Reference Manios, Moschonis and Mavrogianni 73 , Reference Hayes, Duffy and Grady 74 ). The interactions between vitamin D and other micronutrients in dairy-based foods in relation to beneficial effects on bone further underscore the importance of dairy products as a vehicle for vitamin D fortification( Reference Cashman, van den Heuvel and Schoemaker 75 ).
Based on one meta-analysis( Reference Tai, Leung and Grey 42 ) directly comparing the efficacy of dairy products with Ca supplements, dairy products are not better for the BMD of adults than Ca supplements. However, Bischoff-Ferrari et al. ( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 76 ) in their meta-analysis pointed out that a balanced diet including dairy products containing several bone-essential nutrients might be of more importance with regard to building and maintaining BMD than Ca supplements. When pooling the RCT on Ca supplementation by tablets they found no reduction but an increase in hip fracture risk with Ca; in a total of 6504 subjects, the pooled RR was 1·64 (95 % CI 1·02, 2·64). Often Ca intake due to supplement use is considerably higher than the recommended daily allowances. Therefore from a safety perspective( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 76 ) and reported effects on relationships with gastrointestinal symptoms and renal disease( Reference Avenell, Mak and O’Connell 71 ), supplement use may be less preferable then dietary Ca. In addition, increasing dietary Ca intake appears neutral with respect to cardiovascular effects, whereas Ca supplements might raise myocardial infarction risk( Reference Li, Kaaks and Linseisen 77 ). Furthermore, as only two in ten patients effectively comply with Ca+vitamin D prescription after 1 year or more( Reference Castelo-Branco, Cortés and Ferrer 78 ), obtaining bone-relevant nutrients from a balanced diet is in general preferred over the use of supplements.
The role of types of dairy products other than milk for bone mass and fracture risk reduction has so far obtained limited attention. Two epidemiological studies showed that yogurt is also associated with a lower risk for hip fracture in older adults( Reference Sahni, Tucker and Kiel 54 , Reference Sahni, Mangano and Tucker 55 ). Based on nutrient composition and the portions consumed, it is to be expected that cheeses will also contribute to bone health( Reference Cheng, Lyytikäinen and Kröger 79 ). A special feature of cheese is the presence of vitamin K2 ( Reference Beulens, van der and Grobbee 80 ). Vitamin K serves as a cofactor for the microsomal γ-carboxylase which facilitates the post-translational conversion of glutamyl to γ-carboxyglutamyl residues in osteocalcin and numerous other vitamin K-dependent proteins. In its γ-carboxylated state, osteocalcin is a Ca-binding protein in bone, thought to facilitate the mineralisation process( Reference Cashman, van den Heuvel and Schoemaker 75 ).
The overall trend in food consumption in Europe as well as in the USA is to drink less milk and more phosphoric acid-containing soft drinks, which in turn results in a lower dietary Ca:P ratio. If a habitual diet lacks dairy products, the dietary Ca:P ratio will easily drop below the optimal desired level( Reference Kemi, Kärkkäinen and Rita 81 ), which from a bone-health perspective should range from 1:1 to 1·5:1 mg Ca:mg P( Reference Calvo and Tucker 82 ). The short-term cola/meat or cheese studies( Reference Budek, Hoppe and Michaelsen 61 – Reference Kristensen, Jensen and Kudsk 63 ) showed higher serum parathyroid hormone and urinary Ca levels in the first quartile (Ca:P molar ratio≤0·50) v. the other quartiles (Ca:P molar ratio >0·50), therefore underscoring benefits of dairy v. other food products that may decrease the Ca:P ratio. In addition, dietary protein, which is absent in carbonated beverages, stimulates the osteotropic hormone insulin-like growth factor I (IGF-I), which is important for bone formation( Reference Heaney 1 ). Although in the past high protein intake was often assumed to exert a primarily detrimental impact on bone mass and skeletal health, the majority of studies indicate the opposite. A low-protein diet is associated with a higher risk of hip fractures, however, only under conditions of adequate Ca intake( Reference Remer, Krupp and Shi 83 , Reference Mangano, Sahni and Kerstetter 84 ).
Research significance
Current demographic trends leading to an increased number of individuals surviving past the age of 65 years will result in an increased number of osteoporotic fractures( Reference Cauley 85 ). The number of hip fractures potentially can be reduced with dairy products via a beneficial effect on BMC/BMD, especially in the elderly. Drug therapy to reduce facture risk in the elderly is not always feasible but dietary modifications, specifically improving protein, Ca, and vitamin D intakes, may be a more realistic option( Reference Iuliano 86 ). European guidance for the diagnosis and management of osteoporosis in postmenopausal women recommends a daily intake of at least 1000 mg/d for Ca, 800 IU/d (20μg/d) for vitamin D and 1 g/kg body weight of protein for all women aged over 50 years( Reference Rizzoli, Stevenson and Bauer 87 ). However, there is still a high prevalence of Ca and vitamin D insufficiency in women aged 50+ years. In addition, individuals need to consume an overall healthful diet, like increasing plant-based foods or dairy foods, to meet their nutrient requirements( Reference Cifelli, Houchins and Demmer 88 ). What strikes is that there are no permitted claims on individual foods or dietary patterns, although it is well known that some food categories, such as dairy products, provide a number of these bone-relevant nutrients in substantial amounts. The problems that investigators face when trying to establish firm proof for nutrition-related disorders of health have been aptly addressed by Heaney( Reference Heaney 89 ) in his Atwater Memorial Lecture. Most nutrients act in all tissues, all tissues need many nutrients, and inadequate intake impairs many body systems. Nutrients work together, rather than in isolation, and often their effects will not develop when the intakes of other nutrients are suboptimal. Interdependencies among nutrients may well be a part of the explanation for the heterogeneity of results from different research centres and investigators. This ‘orchestra function of nutrition’ needs to be taken into account when addressing the role of dairy products for endpoints of (bone) health. Recent dietary approaches focusing on BMD from a food-group perspective also support the role of dairy products for healthy bones( Reference Van den Hooven, Ambrosini and Huang 90 – Reference Chen, Xiang and Wang 92 ).
Conclusion
Our review showed that (Ca from) dairy products with or without vitamin D positively influences bone mineralisation in children with a low baseline Ca intake, and also in adults. Whether this higher BMD in adults also depends on baseline Ca intake needs further study. Despite the results on BMC, there are currently no studies that have investigated the potential of dairy products to reduce fracture risk in children. In adults, a relatively small meta-analysis on pooled individual data( Reference Kanis, Johansson and Oden 45 ) and a very large meta-analysis( Reference Bischoff-Ferrari, Dawson-Hughes and Baron 44 ) showed that a daily intake of 200–250 ml of milk is associated with a reduction in fracture risk of 5 % or higher. Based on the meta-analyses of two to three RCT that directly compared the efficacy of dairy products with Ca supplements, dairy products are not better for BMD than Ca supplements. Most RCT compared vitamin D-fortified with unfortified dairy products in participants with a high vitamin D status, and therefore we cannot conclude whether vitamin D added to dairy products is more beneficial for bone health. However, several meta-analyses( Reference Avenell, Mak and O’Connell 71 , Reference Lips, Gielen and van Schoor 72 ) that showed that the combination of Ca and vitamin D is better than either of them alone underscore the importance of dairy products as a vehicle for vitamin D fortification. In general, further study on bone health is needed in other ethnicities than Chinese and Caucasians, and in men.
Acknowledgements
We would like to acknowledge Paul T. A. Lips, PhD, Professor Emeritus of VU University Medical Center, the Netherlands who evaluated this paper and provided advice.
The present review received no specific grant from any funding agency, commercial or not-for-profit sectors.
Both authors are employees at FrieslandCampina, a dairy company.







