Introduction
Dementia is increasing in prevalence, driven by global population aging (Prince et al., Reference Prince2015). The condition is characterized by progressive decline in cognition and functional ability. Behavioral and psychological symptoms (also known as neuropsychiatric symptoms) are also common and include depressed or anxious mood, psychosis, and agitation (Finkel et al., Reference Finkel, Costa e Silva, Cohen, Miller and Sartorius1996). Agitation is defined as inappropriate verbal, vocal, or motor activity that is not thought to be caused by unmet need; it encompasses physical and verbal aggression. It is associated with deteriorating relationships with family and professional carers, care home admission, increased care costs, carer burden and burnout, and decreased quality of life (Cummings et al., Reference Cummings2015). Prevalence of agitation in those with dementia is approximately 30% in community-dwelling populations and 40% in care homes (Livingston et al., Reference Livingston2017).
Agitation in dementia has substantial economic consequences, accounting for between 12% and 44% of dementia care costs (Morris et al., Reference Morris2015; Panca et al., Reference Panca2019) and imposing significant costs on unpaid carers (Buylova Gola et al., Reference Buylova Gola2020). Costs rise as severity of agitation increases (Burley et al., Reference Burley, Livingston, Knapp, Wimo, Norman and Brodaty2020; Jones et al., Reference Jones, Aigbogun, Pike, Berry, Houle and Husbands2021; Panca et al., Reference Panca2019). Annual excess health and social care cost of agitation in Alzheimer’s Disease (AD) in the UK is estimated at £2bn (Morris et al., Reference Morris2015).
Evidence from randomized controlled trials (RCTs) of pharmacological interventions for agitation in dementia is mixed. Antipsychotics have limited efficacy but their use is limited by high levels of adverse events, including increased risk of mortality (Langballe et al., Reference Langballe, Engdahl, Nordeng, Ballard, Aarsland and Selbæk2014). Their use is discouraged by regulators, except for very short periods in critical situations. Anti-dementia medications do not seem to help, and there are no data to support the use of anticonvulsants or herbal remedies (Fox et al., Reference Fox2012; Howard et al., Reference Howard2007; Magierski et al., Reference Magierski, Sobow, Schwertner and Religa2020). The antidepressant citalopram, while showing a small positive effect on agitation in dementia in less agitated and less cognitively impaired individuals, is associated with adverse cardiac and cognitive effects which limit its use (Porsteinsson et al., Reference Porsteinsson2014). Anti-depressants have been increasingly prescribed in place of antipsychotics over recent years without an evidence base. Economic evaluations have not featured in pharmacological trials for agitation in dementia. We carried out an RCT of clinical and cost-effectiveness of mirtazapine, one of the most commonly used anti-depressants (Banerjee et al., Reference Banerjee2021). Data from the RCT suggest no clinical benefits in terms of reducing agitation from use of mirtazapine compared with placebo. Here we examine the costs and cost-effectiveness of mirtazapine compared to placebo.
Methods
Data collection
Participants with probable or possible AD and agitation living in the community or care homes were recruited from 26 UK centers. Full details of the sample, measures, and methods have been published elsewhere (Banerjee et al., Reference Banerjee2021). To summarize, eligibility criteria included agitated behaviors that had not responded to non-drug assessment and management (Alzheimer’s Society, 2011); Cohen Mansfield Agitation Inventory (CMAI) score of 45 or more; informed consent; and availability of an informant, either key worker ("paid carer") or family member ("unpaid carer") who consented to participate. Participants were randomized to intervention (mirtazapine) or control (identically encapsulated placebo), stratifying by center and living arrangement (independently in the community or care homes). The study had ethical approval from the Hampshire A South Central Research Ethics Committee (15/SC/0606) and the Medicines and Healthcare products Regulatory Agency.
Data for the economic evaluation were collected alongside other measures by trained researchers (blinded to allocation) from participants and informants at baseline, 6 and 12 weeks. A prospective activity log [adapted from Noble (Reference Noble2012), see also (Marques et al., Reference Marques, Johnson, Gooberman-Hill, Blom and Noble2013)] was distributed to unpaid carers at baseline and 6-week visit for optional use as an aide-memoire during completion of resource use and cost questions at 6- and 12-week interviews. As part of the clinical effectiveness study, data were collected on concomitant medications and trial medication dose modifications. Dosing schedule details are given elsewhere (Banerjee et al., Reference Banerjee2021).
Perspective and horizon
The cost-effectiveness analyses took two perspectives: first that of costs to the health and social care system, and second, since health and care costs are also included, costs to people with dementia and their family carers (societal perspective). As the time horizon within the trial period was 12 weeks, no discounting of outcomes or costs was applied.
Resource use and costs
Health and social care and unpaid care
Comprehensive costs of care for participants with dementia were calculated (including costs of formal/paid care such as that provided by health and social services and also costs associated with unpaid care) using data gathered using the Client Service Receipt Inventory (CSRI) (Beecham and Knapp, Reference Beecham, Knapp, Thornicroft, Brewin and Wing2001) at baseline, 6 and 12 weeks.
Items of resource use were grouped into categories for costing purposes: hospital services, primary and community health, mental health, accommodation (domestic/communal), overnight respite care (in communal settings), community social care, day services, equipment and adaptations (including memory aids), medications, and unpaid care provided to participants. Unpaid care included lost working time (work cut down/given up) and hours of help and support provided by the main carer and family/friends. An abbreviated set of health and social care use questions were asked of unpaid carers; carers were then asked to estimate the proportion of their overall service use attributable to caring for the participant. Carers of participants living at home were also asked to report the number of hours the participant could be left alone in a day and hours of lost sleep due to caring (including assisting the person at night or because of agitation). A yes/no question on carers’ use of prescription and over-the-counter medications was included to assess extent of use but not costed.
The Sussex Partnership Trust Lived Experience Advisory Panel group gave advice on adapting the CSRI for the study. They provided input into question wording and categories of service use for carers; for instance, the group suggested adding counseling services to the list of service questions.
Intervention-specific inputs
Participants could take up to 45 mg of mirtazapine daily. Mirtazapine dosages and frequencies were recorded in the trial database. Safety tests (blood tests and ECG) were excluded from intervention costs as these measures were protocol-driven and not part of routine practice.
Valuation
The base year for prices was 2016/2017. Unit costs (Table S1.1) were taken where available from nationally representative published sources (Curtis and Burns, Reference Curtis and Burns2017; NHS Digital, 2018; NHS Improvement, 2017). The price of generic mirtazapine was taken from NHS Prescription costs analysis (NHS Digital, 2018). Unpaid carer time was valued at opportunity cost in the main analyses [following the lost productivity approach described in Gustavsson et al. (Reference Gustavsson2011) and Wimo et al. (Reference Wimo2013)]. Costs of unpaid care were estimated as either cost of time spent in caring or of time taken off from work to care, whichever was the greater. In estimating the cost of unpaid carer time in caring, those in work were considered to have given up work time (lost production), valued at national average wage (Office for National Statistics, 2017); those not working were considered to have given up leisure time, valued at 35% of national average wage. The CSRI, which was used to estimate carers’ caring hours, covered time spent over the previous week in all caring tasks (including supervision and care home visiting). Unpaid carers chose a time band for hours of care provided per week (ranging from zero hours to 100+ hours per week). A continuous variable for total hours of care was calculated by taking the mid-point of each band. The maximum of the top band was first adjusted to account for nightly sleep time (assumed to be 8 hours if carers reported no lost sleep in caring or the hours remaining once hours of lost sleep were deducted). All time spent in caring tasks received the same valuation rather than (as done in Gustavsson et al. (Reference Gustavsson2011) and Wimo et al. (Reference Wimo2013) attributing a lower value to supervision than hands-on care tasks.
Outcomes
Primary outcome for the economic evaluation was incremental cost per 6-point difference in CMAI score at 12 weeks, from a health and social care system perspective. A 6-point difference represents a clinically significant minimum difference or 30% decrease on the measure from placebo to mirtazapine (Banerjee et al., Reference Banerjee2021). In addition, we conducted a secondary cost-effectiveness analysis on this outcome measure from the societal perspective. We also conducted secondary cost-utility analyses of participants’ and unpaid carers’ quality-adjusted life years (QALYs) from both health and social care and societal perspectives (encompassing health and social care, unpaid care, and out-of-pocket costs of adaptive equipment). Three measures of health-related quality of life were used to derive participant utilities: informant-rated EQ-5D-5L (Dolan et al., Reference Dolan, Gudex, Kind and Williams1995; van Hout et al., Reference van Hout2012), informant-rated DEMQOL-Proxy-U and participant-rated DEMQOL-U (Rowen et al., Reference Rowen2012; Smith et al., Reference Smith2005). Unpaid carers’ utilities were derived from carer self-rated EQ-5D-5L. QALYs were calculated using the area under the curve method, assuming linear change between assessment points (Manca et al., Reference Manca, Hawkins and Sculpher2005).
Cost-effectiveness
Cost per unit of effect of the intervention is known as the incremental cost-effectiveness ratio (ICER). It is calculated as mean difference in costs between mirtazapine and placebo groups (ΔC) divided by the mean difference in outcome (ΔE) between groups.
Mirtazapine would be considered cost-effective if it was significantly more effective and less expensive than placebo (factoring in all relevant costs, not just medication price). The treatment would also be cost-effective if significantly more effective and more expensive than placebo, but where the decision-maker was willing to pay the additional cost (up to a threshold, denoted λ) to achieve the additional effect; or, put another way, if the ICER was below some threshold of willingness to pay for a unit of additional effectiveness, λ (Gray et al., Reference Gray, Clarke, Wolstenholme and Wordsworth2011). The cost-effectiveness decision rule in this case can be expressed as:
Mirtazapine might also be considered cost-effective if significantly less effective and less expensive and the decision-maker considered the sacrifice of some effectiveness worth making to achieve the savings. Mirtazapine would be considered unambiguously not cost-effective if it is both significantly less effective and more expensive.
Incremental net monetary benefit (NMB) is the monetary value of gains in effects associated with the treatment at an assumed value of λ, once the additional cost associated with treatment has been deducted (Drummond et al., Reference Drummond, Sculpher, Claxton, Stoddart and Torrance2015; Gray et al., Reference Gray, Clarke, Wolstenholme and Wordsworth2011). Rearranging the decision rule in (1), NMB is as follows:
Descriptive and cost-effectiveness statistical analyses
Baseline characteristics of participants and carers, service use and costs in the sample with data available at each time-point ("available cases") were summarized by allocation group. Continuous data were described in terms of means (standard errors) and categorical data as numbers (percentages). Numbers completing each item of service use (valid observations) were presented to quantify extent of missing data per item. Group mean costs and outcomes (standard errors) of available cases were compared and mean differences (95% confidence intervals, hereafter known as "confidence intervals") presented. Costs and outcomes of the sample with both data available at baseline and both follow-up points ("complete cases") were summarized by group in terms of means (confidence intervals) alongside their unadjusted differences and model-derived adjusted differences (confidence intervals and p-values).
Multilevel bivariate regressions were estimated for costs and outcomes with fixed effects for baseline cost/outcome and living arrangement at randomization (stratifying variable) and a random effect for center. Multilevel models (MLM) were conducted in Stata 16 (StataCorp, 2019) and estimated by restricted maximum likelihood. Where the sample providing data consisted of 50 or fewer observations, models applied small sample inference for fixed effects and residual denominator degrees of freedom in tests of fixed effects (StataCorp, 2017). NMB over a range of willingness-to-pay values was derived from model estimates, and their 95% confidence intervals were calculated following Fieller’s theorem (Glick, Reference Glick2007; Willan and O’Brien, Reference Willan and O’Brien1996).
There is no societal consensus on what should be paid for a minimum clinically significant difference on the CMAI. A NMB plot and a cost-effectiveness acceptability curve (CEAC) were produced to show the extent to which the primary outcome might be judged cost-effective. The plot of NMB and its confidence limits over a range of willingness-to-pay thresholds illustrates not only the size of any positive values of NMB but also whether the ICER has confidence limits. The point ICER is found where the NMB curve intersects the x-axis (the net benefit for a unit of effect is zero), that is the point where the decision-maker is prepared to pay just the cost of achieving a benefit (Gray et al., Reference Gray, Clarke, Wolstenholme and Wordsworth2011). The confidence limits of the ICER are found where the confidence limits of the NMB curve intersect the x-axis (Drummond et al., Reference Drummond, Sculpher, Claxton, Stoddart and Torrance2015). An unbounded ICER (when the NMB confidence limit lines never intersect the x-axis) indicates that neither the intervention nor the control strategy can be considered to be the more cost-effective (Glick, Reference Glick2007). The CEAC depicts the probability that NMB at a given level of willingness to pay (λ) is greater than zero (Löthgren and Zethraeus, Reference Löthgren and Zethraeus2000). This approach is useful for demonstrating the level of uncertainty associated with deciding whether mirtazapine is cost-effective at different willingness-to-pay values.
For secondary analyses of QALY and health and social care costs outcomes, the ICER and the NMB at £20,000 and £30,000 [the lower and upper limits respectively of the NICE threshold for a QALY gain (National Institute for Health and Care Excellence, 2013)] were calculated and presented alongside descriptive and cost-effectiveness results. Probability of cost-effectiveness over a range of willingness-to-pay thresholds was calculated for narrative commentary in the text.
Sensitivity analyses
Sensitivity analyses (Supplementary file S2) explored impacts on results of varying key assumptions made in the base case for primary and secondary analyses, including accommodation of participants in domestic as well as residential care in total health and social care costs, examining aggregated (EQ-5D-5L) QALYs and costs for the dyad (person with dementia and unpaid carer together), and using an alternative valuation and definition of unpaid carer time.
Missing data
Where possible, efforts to reduce missing data were made. For instance, where information on residential and nursing home stay duration was available but provider sector information was missing, we assumed, in order to assign a unit cost, that residential care and nursing home providers were from the independent sector [almost all such homes in the UK are operated by independent providers (Competition & Markets Authority, 2017)]. However, where a service was reported as having been used but frequency of use was missing, no assumptions were made about the frequency, nor imputation attempted. In this case, the cost of the service would not be calculated, resulting in missing cost information. The extent of missing resource use data was described narratively, comparing valid observations contributing service use data to the expected number of observations at the time-point.
Results
In total, 204 valid trial participants were assessed at baseline and randomized to mirtazapine (N = 102) or placebo (N = 102). At 12-week (6-week) follow-up, 81 (87) people with dementia in the mirtazapine group and 90 (95) in the placebo group continued to participate in the trial. Demographic characteristics were similar across groups at baseline and 12-week follow-up (Table 1), except for the proportion of women. More mirtazapine than placebo participants were female at baseline (74.5% vs 57.8%) and 12 weeks (75.3% vs. 57.8%). At baseline, relatives and friends accounted for the majority of carers (N = 63, 61.2% mirtazapine group; N = 71, 68.9% placebo group); these proportions were similar at 12-week follow-up. Most participants were in care home accommodation at baseline and 12-week follow-up.
Table 1. Baseline characteristics
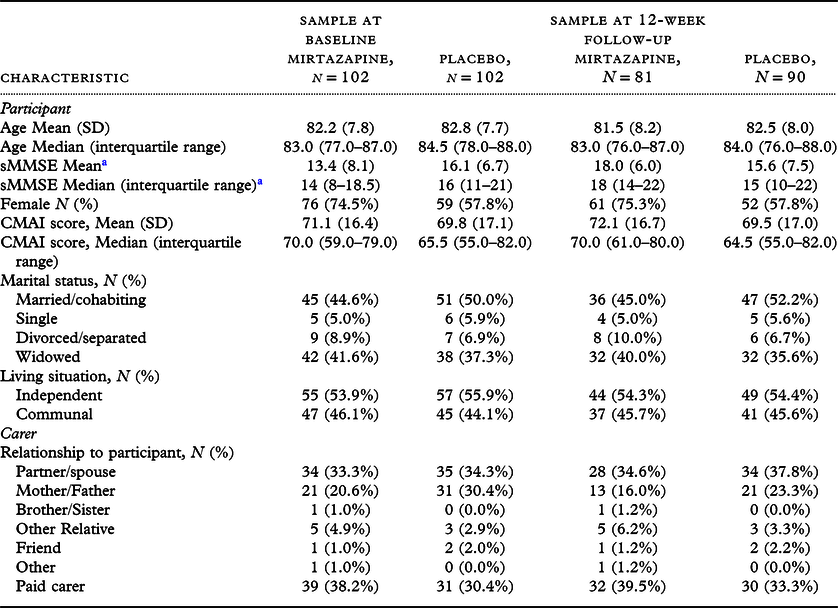
a Baseline: N = 52, Mirtazapine; N = 50, Placebo. At 12-week follow-up: N = 23, Mirtazapine; N = 27, Placebo.
Data were reasonably complete for most service use items (Table S3.1) (96%–100% at baseline, 94%–100% at 6 weeks, 94%–100% at 12 weeks). Data on carers’ care time and service use were similarly complete at baseline (94%–99%) but slightly less so at 6 weeks (87%–90%) and 12 weeks (91%–94%). A filter question in the database classified informants as paid or unpaid carers to determine which carer measures should be completed. A few cases reported to be family/friend carers in the demographics question were classified as paid carers on this question, resulting in loss of unpaid carer resource use data from placebo participants (three cases at baseline; 4 at 6 and 12 weeks).
Table S3.1 sets out paid and unpaid care services used by participants at baseline and follow-ups. Fewer than half of participants used a mental health service in the 12 weeks prior to baseline. Participant use of community and mental health services between 6-week and 12-week follow-up was similar to use between baseline and 6-week assessment. Relatively few participants (15% mirtazapine; 14% placebo) had home care in the pre-baseline period, with means of 2.5 hours and 3.8 hours per week of home care in mirtazapine and placebo groups. In the sample participating at 12 weeks, proportions using home care were similar (11% mirtazapine; 10% placebo) although mirtazapine participants used less than an hour (0.86) while placebo participants used almost 3 hours (2.92) per week in the prior 6 weeks. At baseline, hours provided by unpaid carers greatly exceeded paid home care hours (71 and 60 hours per week in mirtazapine and placebo, respectively). At 12-week follow-up, mirtazapine participants received approximately 80 hours per week of unpaid care, while placebo participants received 56 hours per week.
Looking at carers’ own use of health and support services (Table S3.2), data were fairly complete from carers classified as unpaid (95%–99% at baseline, 90%–94% at 6 weeks, 91%–94% at 12 weeks). More than half used at least one service over the 12 weeks prior to baseline, and approximately half used a service over each follow-up. Carers were asked to estimate the proportion of all services related to their caring role, judging this to be 22% and 23% in mirtazapine and placebo groups respectively at baseline. Estimated proportions were similar at 6 weeks; however, the sample completing 12-week assessments reported divergent estimates (mean 50.8% mirtazapine group, 19.2% placebo group). Carers were also asked whether their care situation had improved since they had used these services and whether their health had been affected as a result of caring (Table S3.3). While groups did not differ on the status of their care situation at baseline or 6 weeks, by 12 weeks, more mirtazapine than placebo carers in receipt of at least one service agreed their situation had improved (12/24 (50%) vs. 4/24 (16.7%), respectively). The proportion of carers reporting that their health was affected by their caring role was at least 50% at each time-point, and proportions were similar between groups. At baseline, carers of people with dementia living at home reported substantial numbers of hours of sleep lost per week as a result of assisting the person or because of the person’s agitation (approximately 8 and 7 hours weekly in mirtazapine and placebo, respectively) (Table S3.4). Hours of lost sleep were similar at 6- and 12-week follow-ups. At baseline, approximately half of the carers in each group reported that the person could be left alone at home. Participants could be left alone for an average of less than 3 hours a day. These estimates were similar at 6 and 12-week follow-ups.
Costs
At baseline, of cases with economic data available, there were no differences between groups in any subcategory of cost, in total health and social care costs or in societal costs of participants with dementia (Table S3.5). Apart from the costs of trial medication, there were no between-group cost differences in the sample participating at 6 weeks. Of those participating in the 12-week follow-up, costs of unpaid care by the dyadic carer over the prior 6 weeks were significantly higher in the mirtazapine than placebo group [difference: £1,120 (95% CI £56, £2,184)]. There were no between-group differences in carers’ health and social care costs (Table S3.6).
Outcome measures
Raw CMAI scores in both groups summarized from available cases’ data were similar at baseline and both follow-ups (Table S3.7). Mean CMAI scores in the sample participating at 6 and 12 weeks (regardless of allocation) were approximately 10 points lower than those in the baseline sample. Raw index scores (utilities) derived from informant-reported quality of life measures were similar between groups. EQ-5D-5L-derived utilities were much lower than those derived from DEMQOL-Proxy-U. Utilities derived from participant-reported DEMQOL-U (completed by less than half the people with dementia participating at each time-point) were somewhat higher than scores on the proxy-completed version in both groups. At each assessment point, carers’ EQ-5D-5L scores were similar between groups (Table S3.8).
Cost-effectiveness analyses
Primary analysis
Mean raw outcome scores and costs of the complete case samples showed no differences between groups (Table 2). Adjusting for baseline measure and living arrangement, the estimate for the difference between groups in both CMAI and costs had wide confidence intervals crossing zero. The point estimate for the ICER on CMAI was negative because costs were slightly lower and outcome slightly better in the mirtazapine compared to placebo group. The NMB curve (Figure 1) shows that net benefit is positive at all willingness-to-pay thresholds from £0 to £30,000: there is monetary benefit once the cost of the intervention has been subtracted. However, the confidence intervals of the NMB curve do not cross zero, illustrating that 95% confidence limits of the ICER could not be defined (see Descriptive and cost-effectiveness statistical analyses), and therefore, neither mirtazapine nor placebo can be judged to be the more cost-effective strategy with a high level of confidence. The CEAC (Figure S3.1) illustrates that probability of cost-effectiveness was 81% at a willingness to pay of £3,000 and 80% at £20,000; also that a 10% confidence interval for the ICER can be defined between willingness to pay of approximately £0 and £3,000 per QALY, giving a low degree of certainty that mirtazapine is cost-effective (see Glick, Reference Glick2007; Gray et al., Reference Gray, Clarke, Wolstenholme and Wordsworth2011).
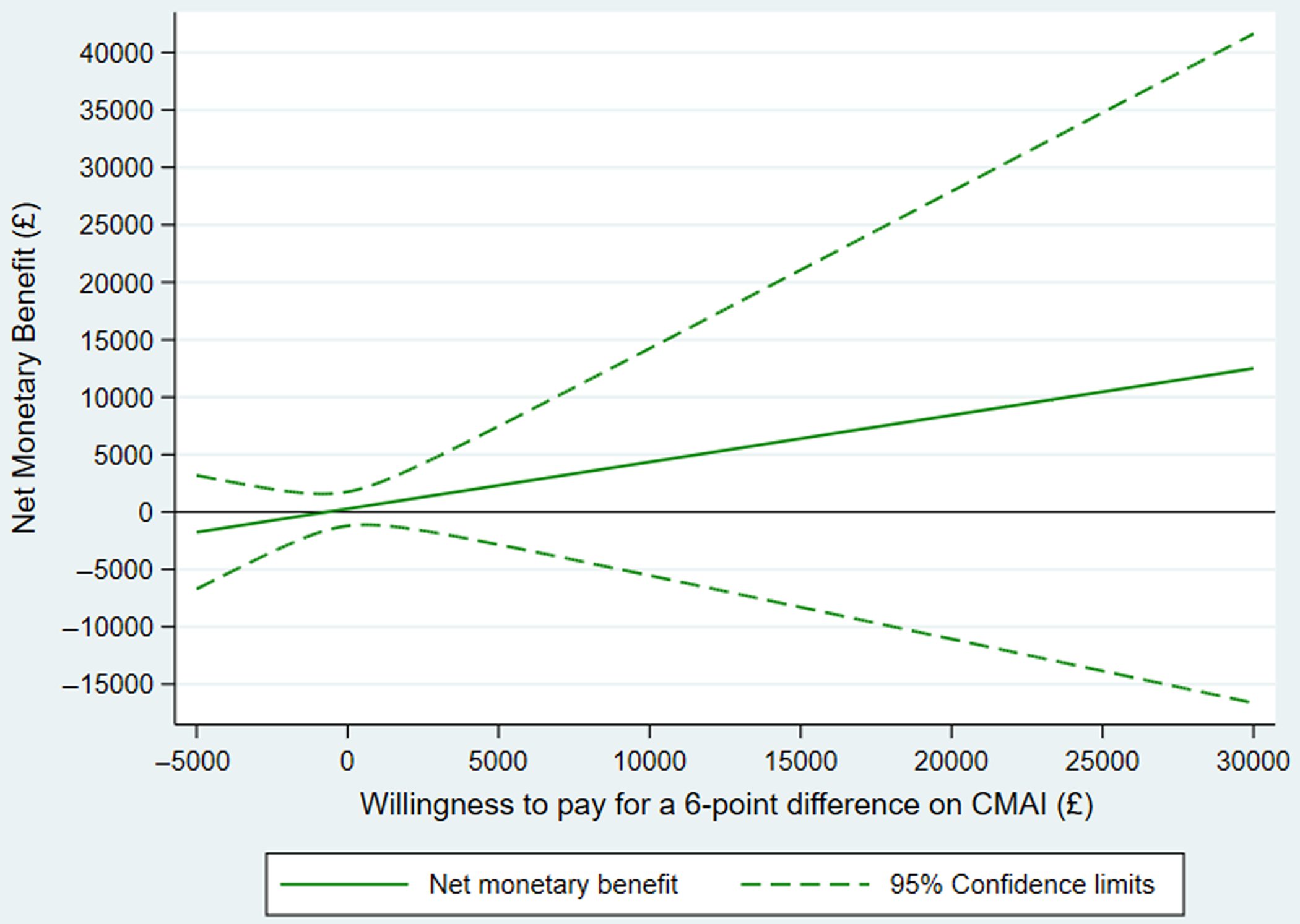
Figure 1. Primary outcome: net monetary benefit plot.
Table 2. Primary outcome/costs: CMAI score and health and social care costs over 12-week study follow-up, raw and adjusted difference between groups and incremental cost-effectiveness ratio
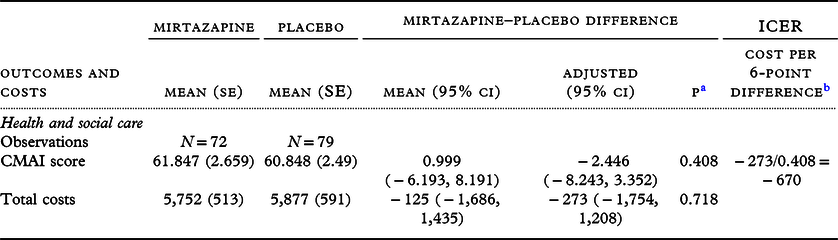
a p-value of the adjusted difference.
b Reversed so that a higher score indicates less agitation and a lower score indicates more agitation.
Secondary analyses
Raw mean outcomes and costs from the health and social care and societal perspectives are presented alongside their raw and adjusted between-group differences, ICER and NMB at £20,000 in Table 3.
Table 3. Secondary participant outcomes and costs over 12-week study follow-up, raw and adjusted difference between groups and incremental cost-effectiveness ratios
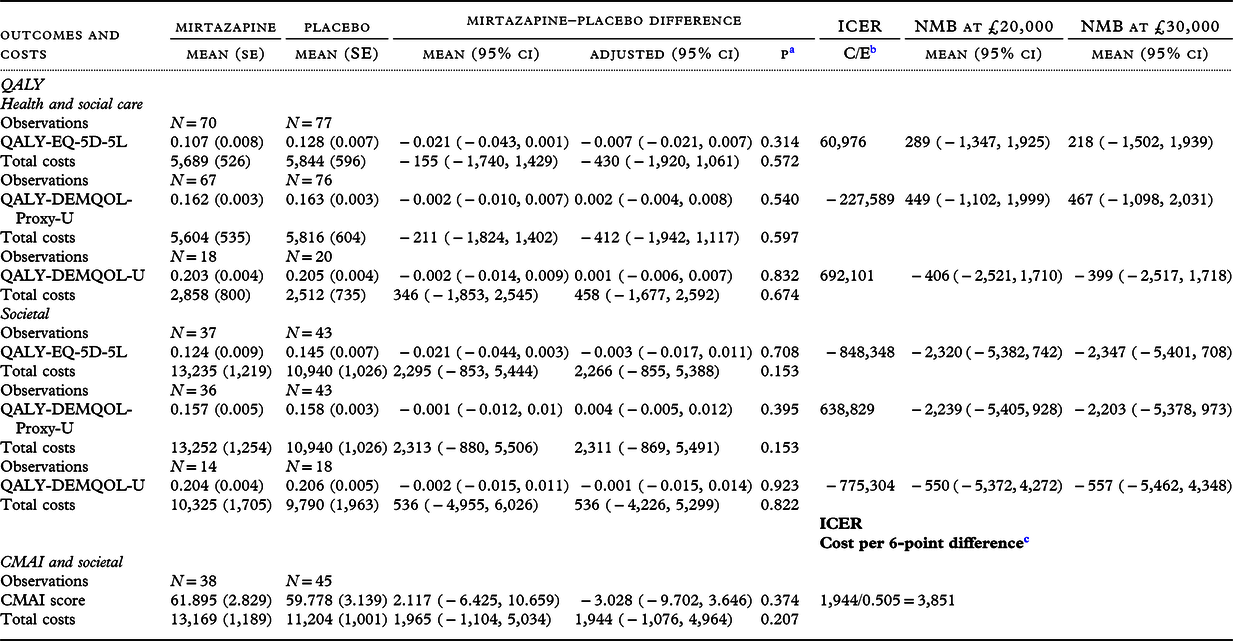
a p-value of the adjusted difference.
b C = mean adjusted cost difference, E = mean adjusted outcome difference.
c Reversed so that a higher score indicates less agitation and a lower score indicates more agitation.
Participant outcomes
Health and social care perspective
On raw participant QALY derived from EQ-5D-5L, DEMQOL-U, and DEMQOL-U-Proxy and costs from a health and social care perspective, there were no differences between groups. Similarly, on adjusted mean differences between groups from the multilevel analyses, there were no differences between groups. Cost-effectiveness results on the DEMQOL-U are not discussed further because of the small numbers involved.
The ICER of EQ-5D-5L-derived QALYs and costs from the health and social care perspective was positive as the sign of the cost difference was negative, and there was a small QALY loss. Results are not discussed further given the latter result.
The ICER from the DEMQOL-U-Proxy was negative as there was a small QALY gain and the sign of the cost difference was negative. Probability of cost-effectiveness ranged from 70% to 72% across a willingness-to-pay range of £0 to £50,000. NMB at the lower NICE threshold (£20,000) was positive, but the 95% confidence intervals crossed zero. The ICER was unbounded, indicating that neither mirtazapine nor placebo could be considered a cost-effective strategy at any willingness to pay to gain a QALY.
Societal perspective
Groups did not differ on costs or CMAI outcomes from the societal perspective. The societal cost per 6-point difference was £3,851, the ICER being unbounded. The NMB at a willingness to pay of £0 was −£1,944 (95% CI −£4,964, £1,076) and at a willingness to pay of £20,000 was £8,150 (95% CI −£14,195, £30,494). Probability of cost-effectiveness ranged between 10% and 77% over this range.
There were no between-group differences in QALYs derived from EQ-5D-5L, DEMQOL-U, or DEMQOL-U-Proxy and costs from the societal perspective, before or after adjustment. Given low numbers on the DEMQOL-U, cost-effectiveness results are not discussed.
The ICER of EQ-5D-5L-derived QALYs and costs from the societal perspective was negative as the sign of the cost difference was positive and there was a small QALY loss. Results are not discussed further for this reason. The ICER from the DEMQOL-U-Proxy was positive and very large; probability of cost-effectiveness ranged from 8% to 10% across willingness-to-pay values from £0 to £50,000.
Carer outcomes
In cases with complete health and support and QALY data, unadjusted and adjusted between-group differences in QALYs were not significant (Table 4). NMB of mirtazapine at £20,000 was negative but the confidence interval crossed zero. The ICER on this measure was unbounded. Results for carers’ societal costs and QALY were similar. Probability of cost-effectiveness from the health and social care perspective did not exceed 45% over willingness-to-pay thresholds ranging between £0 and £50,000 per QALY; probability of cost-effectiveness from the societal perspective did not exceed 19% over the same range.
Table 4. Carer outcomes and costs over 12-week study follow-up, raw and adjusted difference between groups and incremental cost-effectiveness ratios
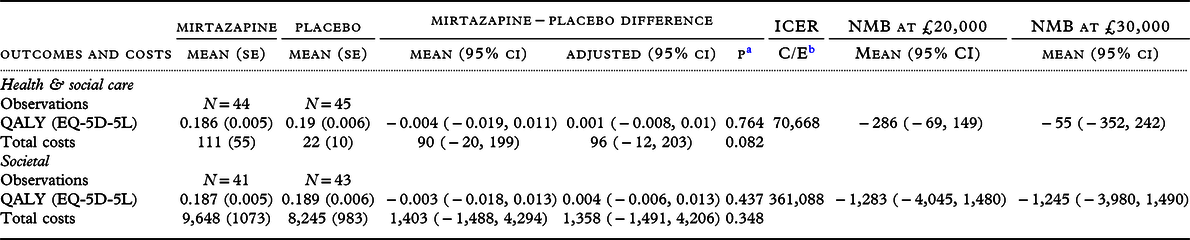
a p-value of the adjusted difference.
b C = mean adjusted cost difference, E = mean adjusted outcome difference.
Sensitivity analyses
Sensitivity analyses of the primary outcome were in line with the base case (Supplementary file S2). On the secondary outcome of participant EQ-5D-5L QALY and societal costs, costs were significantly greater in the mirtazapine group when valuing unpaid carer time at replacement cost. The corresponding NMB of mirtazapine at £20,000 was negative, with negative upper and lower confidence limits, so that costs outweighed the benefit of the intervention. Other sensitivity analyses of secondary outcomes were similar to the base case.
Discussion
On the basis of cost-effectiveness, there is no evidence to support the use of mirtazapine for agitation in dementia. Just as our clinical effectiveness data concluded there was no clinical benefit over placebo, these data are unequivocal in there being no economic reason for mirtazapine being used for treatment of agitation in dementia. The data presented here are novel. While there is economic literature on psychosocial interventions for agitation, to our knowledge no other formal economic analyses have been published of an RCT of an Investigational Medicinal Product (IMP) for agitation in dementia. Consequently, this study provides data that may be of use in subsequent analyses of cost-effectiveness of other IMPs for agitation on dementia.
The mirtazapine group had a marginally lower mean CMAI score than the placebo group at 12-week follow-up. This difference was not statistically significant and much smaller than the pre-specified effectiveness criterion of a 6-point difference in favor of mirtazapine. Groups had similar costs from both health and social care and societal perspectives. On the secondary analyses, groups were similar in both costs and health-related quality of life outcomes. Sensitivity analyses of the primary outcome yielded similar results to the base case. Results of sensitivity analyses of most secondary outcomes were also similar to base-case findings. Between-group differences in societal costs were sensitive to assumptions about valuing unpaid care time. Valuing carer time at replacement cost resulted in mirtazapine being definitely less cost-effective than placebo at willingness to pay per QALY of £20,000.
The clinical effectiveness study (Banerjee et al., Reference Banerjee2021) found no differences between groups in terms of participant neuropsychiatric or cognitive outcomes; however, carers of mirtazapine group participants had worse scores on the Zarit Carer Burden Index at 12 weeks. In the cost-effectiveness study, we found that carers in the mirtazapine group attributed more of their own service use to their caring role and more improvement in their care situation to services used than did carers in the placebo group; also, carers in the mirtazapine group reported more hours of unpaid care at 12 weeks than in the placebo group. Taking these findings together, it is possible that receipt of mirtazapine resulted in increased carer burden and related help-seeking and help-giving.
The substantial costs of caring reported by unpaid carers should be of concern. Carers from both groups lost approximately an hour of sleep each night to care for the agitated person; the mean number of hours they felt able to leave the person alone at home was less than 3 hours. Agitation is a distressing state for people experiencing it and for those around them. Effective strategies for managing agitation and supporting carers are required, tailored to the needs of the person with dementia and their families.
Limitations and strengths
There are limitations to this study, some of which we have discussed elsewhere (Banerjee et al, Reference Banerjee2021). Recruitment beyond February 2020 was constrained by COVID-19, so that only 207 (93%) of the target of 222 were recruited. Importantly for this study, there are potential limits in generalizability resulting from recruitment through mental health services for older people and care homes. Outcomes and costs might have been different for people living in the community treated by primary care services alone. The three main strengths of our study were high follow-up and compliance rates, large sample size, and the broad nature of the study group (in terms of severity of agitation and severity and type of dementia).
This study has reported results based on complete data, as data from participants who withdrew were lost to follow-up or who had died were not available for analysis. In the clinical effectiveness study (Banerjee et al., Reference Banerjee2021), a weak trend to increased mortality was seen in the mirtazapine group, with seven deaths versus one death in the placebo group, although this outcome may have been the result of chance, given the low numbers involved. No differences in other adverse events were detected. We were unable to locate previous trial-based economic evaluations of pharmacological interventions specifically focused on agitation in dementia. The cost-effectiveness of non-pharmacological interventions to manage agitation in care home residents with dementia has been evaluated in trials (Livingston et al., Reference Livingston2019; Romeo et al., Reference Romeo, Zala, Knapp, Orrell, Fossey and Ballard2019) and model-based studies (Livingston et al., Reference Livingston2014). Non-pharmacological management approaches included person-centered care, communication, care mapping and care planning, and combinations of interventions in multicomponent programs, in care home settings (Livingston et al., Reference Livingston2014). A trial of a person-centered care intervention in English care homes was found to be cost-effective in terms of agitation and quality of life, and the intervention was no more costly than usual care from the health and social care perspective (Romeo et al., Reference Romeo, Zala, Knapp, Orrell, Fossey and Ballard2019). Little is known on the effectiveness of any interventions for people with dementia and agitation living in the community (Livingston et al., Reference Livingston2014). There is a need for further research to address this evidence gap. Future trials should include economic evaluations, given the high costs of agitation in dementia (Burley et al., Reference Burley, Livingston, Knapp, Wimo, Norman and Brodaty2020). Evaluators should ensure they collect data on costs to unpaid carers in terms of hours of care, sleep loss, and carer burden.
Conclusion
There is no reason to recommend the use of mirtazapine for people living with dementia who experience agitation on grounds of cost-effectiveness. Effective and cost-effective medicinal management strategies for agitation in dementia are needed, particularly where non-pharmacological approaches have been unsuccessful, and for people with dementia living in community settings.
Acknowledgements
The views and opinions expressed here are those of the authors and do not necessarily reflect those of the HTA program, NIHR, National Health Service, or the Department of Health and Social Care. We thank all the participants and carers that gave their time to be part of this study; Antony Colles, Martin Pond, and the NCTU data management team for database design and expertise; members of the SYMBAD data monitoring committee and trial steering committee (Bart Sheehan [chair DMC], Peter Connelly [chair TSC], Adrian Treloar, Siobhan Creanor, Toby Prevost, Andy Barker, Chris Penrose, and Julie West); Julia Fountain and our Lived Experience Advisory Forum; the Alzheimer’s Society for providing patient and public involvement support into the study; the NIHR Clinical Research Network Dementia specialty for help in recruitment; Join Dementia Research registry team; Antony Walsh and the sponsorship and grant management teams at the University of Sussex; and referring clinicians in every area. SB is supported as an NIHR Senior Investigator. RH is supported by the UCLH NIHR BRC. GL is supported by the UCLH NIHR BRC, North Thames NIHR Applied Research Collaboration, and as an NIHR Senior Investigator. AT is supported by Newcastle NIHR BRC and Brains for Dementia Research. JOB is supported by the Cambridge NIHR BRC.
Declaration of interests
SB reports personal fees and non-financial support from Lilly, personal fees from Boehringer-Ingelheim, personal fees from Axovant, personal fees from Lundbeck, personal fees from Nutricia, and honoraria from the Hamad Medical Service for lectures and talks, outside the submitted work; he is a Trustee of the Alzheimer’s Society and has research grants from NIHR, ESRC, and ESPRC. AB reports being National Clinical Director for Dementia at NHS England and receiving professional fees from NHS England, personal fees from International Journal of Geriatric Psychiatry, personal fees from lectures and talks, personal fees from medico-legal reports, and the Driver and Vehicle Licensing Authority, outside the submitted work. CB reports grants and personal fees from Acadia pharmaceutical company, grants and personal fees from Lundbeck, personal fees from Roche, personal fees from Otsuka, personal fees from Novartis, personal fees from Eli Lilly, personal fees from Suven, personal fees from Sunovion, personal fees from ADDEX, personal fees from Exciva, personal fees and other from Synexus, personal fees and other from Novo Nordisk, other from Biogen, outside the submitted work. PB reports work as a paid Consultant for TauRx Therapeutics outside the submitted work. RH reported grant support from NIHR and being a Trustee of Alzheimer’s Research UK. J O’B reports personal fees from TauRX, personal fees from Axon, personal fees from GE Healthcare, personal fees from Eisai, non-financial support from Alliance Medical, personal fees from Roche, grants from Merck outside the submitted work. NT reports grant support from Avenir Pharma and NIHR ARC and CRN leadership roles. AT reports grants from NIHR HTA, during the conduct of the study. MK reports grants from NIHR outside the submitted work. All other authors report no relevant interests other than NIHR funding for investigator time on this grant.
Source of funding
This project was funded by the UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) program (project number 13/115/76).
Description of authors’ roles
SB was the chief investigator for the study and designed and managed the study with input from the group. CH carried out the economic analyses for this paper; SS and LS carried out the prior statistical analyses. All authors had access to data and participated in data interpretation. JH, SS, LS, CH, and SB have verified the underlying data. CH drafted the first and subsequent versions of this paper with input and key revisions by all authors, who reviewed and approved the final submitted paper.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1041610222000436









