INTRODUCTION
The dystrophinopathies are X linked muscle diseases primarily affecting males and range in severity due to the abnormal expression of the protein dystrophin. Various dystrophin isoforms are expressed in different cell types, including nerve and glial cells in the brain. In normal brains according to both autopsy and animal studies, dystrophin has been localized to areas of the cortex, hippocampus, and cerebellum (Anderson, Head, Rae, & Morley, Reference Anderson, Head, Rae and Morley2002). The role of dystrophin in the central nervous system (CNS) is still unclear, but abnormal dystrophin likely influences brain circuitry affecting the development of brain structures (Gorecki, Lukasiuk, Szklarczyk, & Kaczmarek, Reference Gorecki, Lukasiuk, Szklarczyk and Kaczmarek1998; Kim, Wu, & Black, Reference Kim, Wu and Black1995; Lidov, Reference Lidov1996; Sogos, Curto, Reali, & Gremo, Reference Sogos, Curto, Reali and Gremo2002) and function (Anderson, Head, & Morley, Reference Anderson, Head and Morley2003, Reference Anderson, Head and Morley2004). Altered or absent dystrophin may cause a cascade of molecular abnormalities affecting cell stabilization, protection from damage, and regulation in signaling pathways (Allen, Whitehead, & Froehner, Reference Allen, Whitehead and Froehner2016).
Neurodevelopmental difficulties are common in the disorder. The absence of, or incomplete isoforms of, dystrophin have been hypothesized to impact brain functioning (Anderson, Head, & Morley, Reference Anderson, Head and Morley2012; Mehler, Reference Mehler2000). Neurocognitive evidence suggests areas of specific impairment rather than generalized deficits. Although there is an increased risk of overall intellectual impairment, most children with dystrophinopathy have average intellectual functioning (Cotton, Voudouris, & Greenwood, Reference Cotton, Voudouris and Greenwood2001).
There is evidence that cognitive deficits in dystrophinopathy are related to the molecular abnormalities in the disorder. A variety of mutations in the dystrophin gene alters expression of CNS dystrophin isoforms. Full-length dystrophin (Dp427) as well as other dystrophin isoforms including Dp71 and Dp140 are normally found in the brain and their absence is negatively associated with cognitive abilities (Bardoni et al., Reference Bardoni, Felisari, Sironi, Comi, Lai, Robotti and Bresolin2000; Daoud, Angeard, et al., Reference Daoud, Angeard, Demerre, Martie, Benyaou, Leturcq and Tuffery2009; Waite, Brown, & Blake, Reference Waite, Brown and Blake2012. Mutations affecting Dp71), typically distal of exon 63, and Dp140, localized to exon 44–45, are associated with a greater frequency of intellectual impairment (Daoud et al., Reference Daoud, Angeard, Demerre, Martie, Benyaou, Leturcq and Tuffery2009; Lenk, Hanke, Thiele, & Speer, Reference Daoud, Angeard, Demerre, Martie, Benyaou, Leturcq and Tuffery1993; Moizard et al., Reference Moizard, Toutain, Fournier, Berret, Raynaud, Billard and Moraine2000; Taylor et al., Reference Taylor, Betts, Maroulis, Gilissen, Pedersen, Mowat and Buckley2010; Tuffery et al., Reference Tuffery, Lenk, Roberts, Coubes, Demaille and Claustres1995).
Ricotti et al. (Reference Ricotti, Mandy, Scoto, Pane, Deconinck, Messina and Muntoni2016) found an increased prevalence of cognitive and behavioral deficits within individuals with specific downstream gene mutations (Ricotti et al., Reference Ricotti, Mandy, Scoto, Pane, Deconinck, Messina and Muntoni2016). Our research group discovered that downstream mutations are linked to lower intelligence quotients (IQ) as well as reduced digit forward span (Fee et al., 2017, in submission), a proposed measure of verbal span capacity (Hinton, De Vivo, Fee, Goldstein, & Stern, Reference Hinton, De Vivo, Fee, Goldstein and Stern2004). The frequency and severity of cognitive dysfunction thus appears to be related to mutations affecting more distal areas of the gene, located downstream from exon 43.
Performance on academic measures in the dystrophinopathies is generally lower than expected, with most children falling below expected age and grade levels (Billard et al., Reference Billard, Gillet, Signoret, Uicaut, Bertrand, Fardeau and Santini1992; Hendriksen & Vles, Reference Hendriksen and Vles2006; Hinton, De Vivo, Nereo, Goldstein, & Stern, Reference Hinton, De Vivo, Nereo, Goldstein and Stern2001; Hinton, DeVivo, Fee, Goldstein, & Stern, Reference Hinton, De Vivo, Fee, Goldstein and Stern2004; Leibowitz & Dubowitz, Reference Leibowitz and Dubowitz1981; Worden & Vignos, Reference Worden and Vignos1962). Moreover, academic scores of children with dystrophinopathy are depressed relative to nonverbal IQ, and when compared to unaffected siblings across reading, spelling and math (Hinton et al., Reference Hinton, De Vivo, Fee, Goldstein and Stern2004).
There is substantial evidence for an increased rate of reading disabilities and delays in developing reading skills and poor spelling abilities (Astrea et al., Reference Astrea, Pecini, Gasperini, Brisca, Scutifero, Bruno and Battini2015; Billard, Gillet, Barthez, Hommet, & Bertrand, Reference Billard, Gillet, Barthez, Hommet and Bertrand1998; Dorman, Hurley, & D’Avignon, Reference Dorman, Hurley and D’Avignon1988; Fabbro et al., Reference Fabbro, Marini, Felisari, Comi, D’Angelo, Turconi and Bresolin2007; Hendriksen & Vles, Reference Hendriksen and Vles2006; Hinton et al., Reference Hinton, De Vivo, Fee, Goldstein and Stern2004). There is less research examining computational math skills, but our studies found performance to be weak when compared to nonverbal IQ and unaffected siblings (Hinton et al., Reference Hinton, De Vivo, Nereo, Goldstein and Stern2001, Reference Hinton, De Vivo, Fee, Goldstein and Stern2004). Overall, across studies, individuals with dystrophinopathies have generalized weakness on tests of academics.
Executive functioning deficits may underlie impaired academic achievement evident in children with dystrophinopathies. There is a wide range of cognitive functions ascribed to executive tasks; the ability to focus and shift attention, organize and plan assignments, and problem solve are essential for attaining academic goals. Executive skills have been strongly associated with academic performance in healthy children (Best, Miller, & Naglieri, Reference Best, Miller and Naglieri2011), wherein specific executive abilities such as inhibition, shifting, and working memory have been linked to performance in specific academic subject areas (St Clair-Thompson & Gathercole, Reference St Clair-Thompson and Gathercole2006). Working memory and planning (Cutting, Materek, Cole, Levine, & Mahone, Reference Cutting, Materek, Cole, Levine and Mahone2009; Sesma, Mahone, Levine, Eason, & Cutting, Reference Sesma, Mahone, Levine, Eason and Cutting2009), shifting (van der Sluis, de Jong, & van der Leij, Reference van der Sluis, de Jong and van der Leij2007), and cognitive flexibility (Welsh, Nix, Blair, Bierman, & Nelson, Reference Welsh, Nix, Blair, Bierman and Nelson2010) have been shown to predict reading skills.
Executive skills are also associated with the development of math skills (Clark, Pritchard, & Woodward, Reference Clark, Pritchard and Woodward2010; Mazzocco & Kover, Reference Mazzocco and Kover2007), and working memory more specifically has been predictive of math achievement (DeStefano & LeFevre, Reference DeStefano and LeFevre2004; Geary, Hoard, Byrd-Craven, Nugent, & Numtee, Reference Geary, Hoard, Byrd-Craven, Nugent and Numtee2007; Swanson & Sachse-Lee, Reference Swanson and Sachse-Lee2001). Even over time, working memory capacity determined by performance on span tasks has been linked to both mathematics and science skill development (Gathercole, Pickering, Knight, & Stegmann, Reference Gathercole, Pickering, Knight and Stegmann2004).
With respect to children with dystrophinopathy, although our prior work has shown that IQ and digit span performance contributed to academic achievement (Hinton et al., Reference Hinton, De Vivo, Fee, Goldstein and Stern2004), the role of other executive skills has not yet been thoroughly explored. In a large cohort of children with dystrophinopathy, contributions of both forward and backward digit span were shown to be associated with reading performance, with forward span having the greatest contribution (Leaffer, Fee, & Hinton, Reference Leaffer, Fee and Hinton2016).
Although we have argued that performance on digit span may reflect an underlying “core deficit” in children with dystrophinopathy (Cyrulnik et al., Reference Cyrulnik, Fee, Batchelder, Kiefel, Goldstein and Hinton2008; Hinton et al., Reference Hinton, De Vivo, Fee, Goldstein and Stern2004), others have suggested more generalized executive skill deficits (Donders & Taneja, Reference Donders and Taneja2009; Mento, Tarantino, & Bisiacchi, Reference Mento, Tarantino and Bisiacchi2011; Wicksell, Kihlgren, Melin, & Eeg-Olofsson, Reference Wicksell, Kihlgren, Melin and Eeg-Olofsson2004). We recently documented generalized deficits across multiple areas of executive functioning in children with dystrophinopathy (Fee et al., 2017, in submission). Surprisingly, however, only performance on forward digit span, and no other measure of executive functioning, was associated with mutation position.
In addition to executive functioning abilities, several other factors may contribute to academic performance. First, IQ is strongly associated with academic achievement (Mayes, Calhoun, Bixler, & Zimmerman, Reference Mayes, Calhoun, Bixler and Zimmerman2009; McGrew, Flanagan, Keith, & Vanderwood, Reference McGrew, Flanagan, Keith and Vanderwood1997). Second, demographic variables such as socioeconomic status (SES) and parental education are associated with academic performance (Bradley & Corwyn, Reference Bradley and Corwyn2002; Duncan & Murnane, Reference Duncan and Murnane2011). Third, behavioral indicators (prosocial behavior, motivation, and the extent of individual academic engagement) (Komarraju & Nadler, Reference Komarraju and Nadler2013; Wentzel, Reference Wentzel1993), have been shown to be associated with both academic performance, and the classroom environment for learning (Wang & Eccles, Reference Wang and Eccles2013). Fourth, chronic illness is also associated with academic performance (Taras & Potts‐Datema, Reference Taras and Potts‐Datema2005). Fifth, physical limitations may limit school interactions as well as attendance, thus influencing individual academic functioning (Pinquart & Teubert, Reference Pinquart and Teubert2011). Among children with dystrophinopathies, any of these factors might play a significant role in academic achievement.
Most studies examining academics in the dystrophinopathies have used homogenous samples that lacked diversity, failing to consider the multiple factors that contribute to academic performance including cognitive ability, educational opportunity, SES, motor deficits, and the psychosocial stressors of having a physical disability. Additionally, the relationship between mutation position and academic skills has not been explored. To clarify specific predictors of individual performance, the contributions of generalized executive deficits as well as other individual factors including IQ, physical limitations, mutation position, behavior and SES were examined with respect to real world functioning, as measured by academic skill level in a diverse sample of 50 boys with dystrophinopathy.
Given prior evidence, we hypothesized that academic skills in the present sample would: (1) be lower than normative data from similar-aged children; (2) be more impaired in boys with downstream genetic mutations than those with upstream mutations; (3) share significant variance and overlap with executive deficits (selective attention/inhibitory control, set-shifting, working memory and processing speed) including digit span. Exploratory analyses examined whether illness severity, SES, IQ, behavioral variables, and demographic variables also impacted academic performance.
METHODS
Sample
Boys (n=50) were included given the nature of the disorder and the prevalence of dystrophinopathy among females is quite rare. Participants were enrolled from the Pediatric Neuromuscular Center and Muscular Dystrophy Association (MDA) clinic located at New York Presbyterian Hospital, the academic hospital associated with Columbia University. The clinic population came from the greater New York City (NYC) metropolitan area and a wide range of SES levels. Inclusion criteria included a genetically confirmed diagnosis of dystrophinopathy (both Duchenne muscular dystrophy [45 participants] and Becker muscular dystrophy [5 participants] were included) and between 5 and 17 years of age. Those potential participants not proficient in English were excluded from the study because of the use of measures normed on English speaking samples. All other participants who were capable of giving assent, spoke English as a primary language (not of parents), an interest in research participation, and in relatively good health with no comorbid medical diagnosis apart from the diagnosis of dystrophinopathy were included.
Sixty-four percent of the sample was receiving glucocorticoid therapy at time of participation for treatment, but no other reported medication. On a self-report parent measure, 14% (seven participants) were described as having attention deficit hyperactivity disorder, 4% (two participants) a Spectrum Disorder, and 32% (16 participants) were generally described as having developmental delay (including language). Thirty-six percent (18 participants) were bilingual, but considered English as their primary language.
Procedures
The study was approved by the institutional review board (IRB) at Columbia University Medical Center (IRB #AAAA5627) and The Graduate Center of City University of New York and was supported by a MDA grant. After an introduction by the treating physician, interested participants were described protocol details by the study coordinator. All parents or guardians gave informed consent and all participants gave assent before enrollment.
Evaluations were conducted in a quiet room free of potential distractions, and breaks were provided; assessment duration was approximately 2 hr. Neuropsychological measures were administered to all children in a standardized order, chosen to assess a broad range of intellectual function that emphasized attention/executive skills and minimized potential confounding effects of impaired motor abilities. Data were scored and converted to standardized scores using normative data. Additionally, participant medical records were reviewed for the results of genome sequencing and dystrophin gene mutation analysis, and mutation position was recorded for each participant. Data were coded without links to identifying information, and entered into a secure database; files were then stored in a locked file cabinet. Patient confidentiality was protected and ensured by the Health Insurance Portability and Accountability Act, Institutional Review Board regulations, and the study team.
Genetic Mutation Groups
Mutation positions were categorized into two groups: upstream of exon 43 and downstream of exon 43 (including Dp 140 and Dp 71).
Assessment Measures
Standardized neuropsychological measures with strong normative data were selected for the assessment. Measures were carefully chosen to minimize the amount of motor skills needed and most were able to be answered with a verbal response or by pressing a computer button.
IQ composite
Participants completed two measures that were used as proxies for general IQ: The Peabody Picture Vocabulary Test-IV (PPVT-IV) (Dunn & Dunn, Reference Dunn and Dunn2007), a measure of single word comprehension, and the Comprehensive Test of Nonverbal Intelligence-II (CTONI-II), a nonverbal measure of analogical reasoning, categorical classification, and sequential reasoning. Both measures are highly correlated with the Full Scale IQ score of the Wechsler Intelligence Scales for Children with ranges from .69 to .88 (Rossen, Shearer, Penfield, & Kranzler, Reference Rossen, Shearer, Penfield and Kranzler2005). Raw scores were age-standardized and converted to z scores. Scores on the PPVT-IV and CTONI-II were combined and the mean was used to create a composite IQ score for analysis.
Academic function
The Woodcock-Johnson III Tests of Achievement (Woodcock, McGrew, & Mather, Reference Woodcock, McGrew and Mather2001) is a comprehensive and extensively used test battery for assessing academic achievement skills in individuals aged 2 to 90 years and normed on diverse communities in the U.S. population. The measure has strong reliability and validity, possessing correlations ranging from .60 to .70 with other academic and IQ measures. The subtests of Single Word Reading, Spelling and Calculation were calculated for each child and the Total Academic score is composed of the combined performance on the subtests. Age-standardized scores were used in analysis.
Executive skills
Selected subtests from The National Institutes of Health (NIH) Toolbox were chosen given the assessment’s wide use among diverse populations and representative normative data for individuals between ages of 3 to 85 in the U.S. population. Subtests from the Cognitive Toolbox (Hodes, Insel, Landis, & NIH Blueprint for Neuroscience Research, Reference Hodes, Insel and Landis2013; Weintraub et al., Reference Weintraub, Bauer, Zelazo, Wallner-Allen, Dikmen, Heaton and Gershon2013) were administered, including attention (Flanker Inhibitory Control Test), set-shifting (Dimensional Change Card Sort Test), working memory (List Sorting Working Memory Test), and processing speed (Pattern Comparison Processing Speed Test). These measures possessed short administration times and normative data matched the study sample.
First, the Flanker task required the participant to focus on a stimulus in the midst of congruent and incongruent stimuli, recording both reaction time (RT) and accuracy. Second, the Dimensional Change Card Sort Test measured set-shifting by assessing two dimensions, both the color and shape of an object; moreover, cognitive flexibility was evaluated by having the individual shift sets by dimensions, recording RT and accuracy. Third, the List Sorting Working Memory Test measured span and sequencing of visually and orally presented stimuli (animals and foods). The participant organized the items in size order from smallest to largest, first within a single dimension and then on two dimensions. The score was equal to the number of items recalled and sequenced correctly. Fourth, the Pattern Comparison Processing Speed Test measured processing speed. Participants had 85 s to rapidly judge as many item sets as possible (whether two pictures presented were the same or different).
Additionally, the Digit Span subtest from the Wechsler Intelligence Scale for Children-IV (Wechsler, 2004), was administered as a measure of verbal span and working memory. Participants were asked to repeat numbers in the same order, or in the reverse order, of presentation. Total score reflects performance on the entire measure, and individual maximum span length of both forward and backward administration was also calculated.
Motor function
The Brooke and Vignos Scale was used to assess motor functioning (Brooke et al., Reference Brooke, Griggs, Mendell, Fenichel, Shumate and Pellegrino1981; Vignos, Spencer, & Archibald, Reference Vignos, Spencer and Archibald1963). The scales include measurements of upper and lower extremity functioning. Scores were categorized into minimal impairment (1–5); able to walk, climb stairs, and full use of arms, moderate (6–12); able to walk, but unable to climb stairs and not able to raise hands over head; and severe impairment (13–16); wheelchair bound, limited use of upper extremities. Fine motor abilities were also assessed using a finger tapping task to ensure participants were able to respond accurately using the computer keyboard for the NIH Toobox measures. Each motor measure was administered by, or supervised by, a licensed physical therapist in the MDA clinic. For regression analysis, upper and lower extremity motor scores were combined for analysis. Individuals with a gross motor score of 13–16 and finger tapping less than −1.5 standard deviation from the mean were categorized as severely impaired.
SES
Family income was reported by parents in a family history questionnaire by checking off a range of incomes provided for the household. Income was based on reported household income and number of individuals in the home and then compared to the NYC census report for annual median household income; income was categorized into low, middle, high-income levels. Income was recoded for analysis comparing low to middle/high income.
Behavior
Parental report behavior measures were examined for potential contributions to cognitive performance. The Behavior Assessment System for Children, second edition (BASC-2) (Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004), is a multidimensional assessment that evaluates various aspects of behavior and personality from the perspective of the parent. The BASC-2 was used to determine overall behavioral issues both externalizing problems (attention difficulties, conduct problems, hyperactivity) and internalizing problems (anxiety, depression, and somatization) by using the Behavioral Symptoms Index (BSI) score. The BASC-2 yielded a T-score for the BSI score. Adverse outcome was coded as a score of T > 67 on the scale and dichotomized (elevated versus within normal limits).
Additionally, the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia, Isquith, Guy, & Kenworthy, Reference Gioia, Isquith, Guy and Kenworthy2000) was used to assess executive function and self-regulation in everyday life and was examined as a potential marker for deficits in attention/executive skills. The scale is designed for children 5 to 18 years of age and has been well standardized. The measure provided information on different aspects of executive functioning through an overall Global Executive Composite score (GEC). T-Scores were generated for the composite scale and a T score >65 was categorized as elevated. The BRIEF GEC composite score was dichotomized into elevated or within normal limits and used for analysis.
Statistical Analysis
Descriptive demographic analyses were based on standardized scores. Shapiro-Wilk analyses were run to ensure the data were normally distributed for parametric analyses and Levene’s test assessed for homogeneity of variances. For regression analysis, variables were entered into a correlation matrix to determine their association with each other. Multivariate outliers were investigated by examination of Mahalanobis Distance and predicted residual values. Multicollinearity was examined by inspecting the correlation coefficients and tolerance/VIF values. Independence of observations was determined by the Durbin-Watson statistic. Independence of errors from regression predictors was confirmed by histogram evaluation and P–P plot of standardized residuals.
Within-subject analysis
Paired t tests were conducted across participants to compare performance on each academic measure (reading, spelling, calculation, total) with individual IQ composite estimates. Alpha was set at .05.
Group analysis
Welch’s test (independent sample t test for unequal variances and sample sizes) was conducted between mutation groups (downstream of exon 43: n=32; upstream of exon 43; n=18) for the following dependent measures of academic performance: reading, spelling, calculation, and total. Alpha was set at .05.
Regression analysis
Four linear regressions were computed for the following outcome variables indexing academic performance: (1) reading, (2) spelling, (3) calculation, and (4) total. Frequencies and distributions of each variable were determined and each was entered into a correlation matrix to determine whether it was associated with the outcome variable of interest. Significant independent variables were entered as predictors in two steps. Executive function measures including digit span forward and backward were included in the first block of the regression model (model I). The motor, demographic, SES, IQ, mutation position, and behavior were then added to the overall regression model in step two (model II). Standardized beta values (β) were used to indicate the relative influence of each predictor. Alpha was set at .01 to reduce the probability of Type I error.
RESULTS
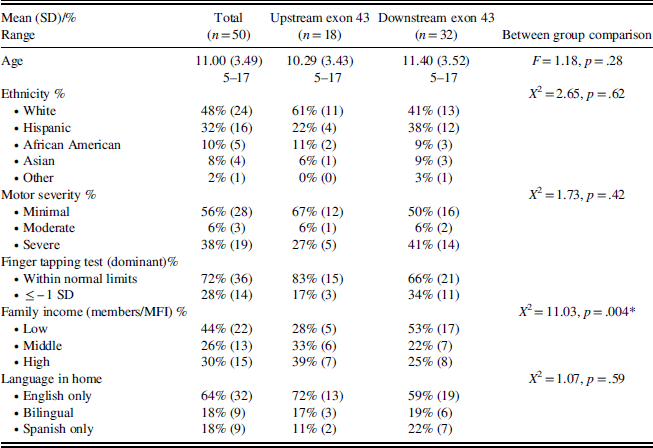
Table 1 illustrates demographic information for the sample, demonstrating that families who agreed to participate came from diverse backgrounds with a broad range of SES and education.
Table 1 Descriptive characteristics of the total sample
* p<.01.
Within-Subject Analysis
Across participants, reading, calculation, and total academic scores, although well within normal limits, were lower than expected when compared to IQ (Table 2).
Table 2 Neuropsychological mean performance standardized data

*p<.05.
PPVT-IV=Peabody Picture Vocabulary Test-IV; CTONI-II=Comprehensive Test of Nonverbal Intelligence-II.
Group Analysis
Demographic information between groups was not significantly different except for SES; more boys with downstream mutations came from low-income families (Table 1). As demonstrated in other studies, participants with mutations downstream of exon 43 had greater intellectual impairments (F(1,48)=7.05; p=.01). Boys with a downstream mutation also exhibited significantly poorer performance on reading, spelling, calculation, and total academic measures than boys with an upstream mutation (Figure 1).
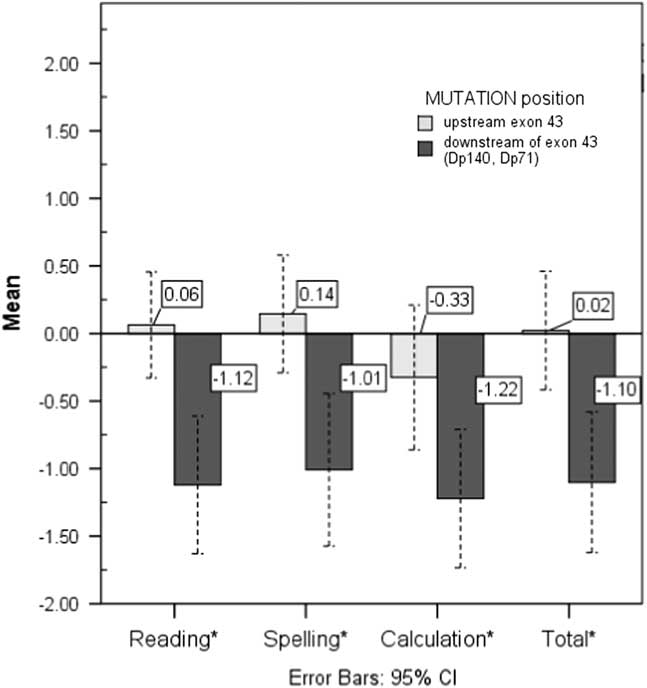
Fig. 1 Between-mutation position group comparisons on academic performance. *= p <.05; effect sizes, partial n 2: reading =.19, spelling = .15, calculation =.11, total = .16.
Regression Analysis
Of note is that only 44 of the 50 participants originally enrolled had completed parent questionnaire measures. Of those, 35 completed them in English and 9 completed them in Spanish, based on the parent or guardian’s primary language. To ensure that those participants whose parents did not compete the questionnaires were comparable to those whose parents did complete the scales, multiple exploratory independent sample t tests were run. Group age, IQ, motor abilities, income, behavioral measures, mutation position, and performance on all cognitive measures did not differ between the six families whose scales were not completed and those with completed scales, suggesting the missing data were reflective of the overall sample (Table 3).
Table 3 Characteristics of the sample for regression analysis (n=44)
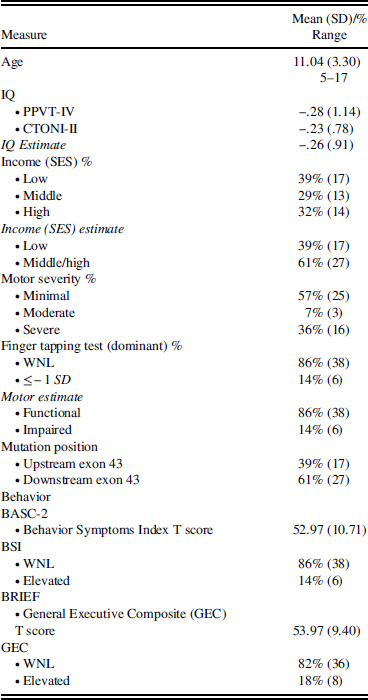
PPVT-IV=Peabody Picture Vocabulary Test-IV; CTONI-II=Comprehensive Test of Nonverbal Intelligence-2; WNL=Within Normal Limits; BASC=Behavior Assessment System for Children-2; BSI=Behavior Symptoms Index; BRIEF=Behavior Rating Inventory of Executive Functioning; GEC=Global Executive Composite
Regression assumptions were met for subsequent analyses, wherein no standardized residual outliers >2.5 standard deviations were present, there was no evidence of multicollinearity, errors were independent and normally distributed, and relationships between predictor and outcome variables were linear and homoscedastic.
The regression model for the cognitive executive measures (model I) was significant (F(6,38)=5.71; p=.001) with digit span forward as the only significant predictor of total academic achievement in the model (β=.35; t(44)=2.55; p=.009). For total academic achievement, the overall model (model II) was significant, F(12,32)=4.02; p=.003, with predictors accounting for 53% of the variance; however, digit span forward was again the only significant predictor in the model, with better performance predicting higher total scores (β=.45; t(44)=2.45; p=.001).
For individual academic areas, the overall models were also significant. Model I was significant for all subject areas, reading (F(6,38)=4.60; p=.001); R 2 Adjusted =.41), spelling (F(6,38)=3.12; p=.005); R 2 Adjusted =.41) and calculation (F(6,38)=6.13; p=.000); R 2 Adjusted =.54); greater digit span forward significantly predicted better spelling (β=.35; t(44)=2.48; p=.008) and better calculation (β=.38; t(44)=2.65; p=.009) performance. Both digit forward (β=.37; t(44)=3.00; p=.003 ) and digit backward (β=.35; t(44)=2.52; p=.009) significantly predicted reading. For model II, all predictors significantly predicted reading (F(12,32)=4.32; p=.001); R 2 Adjusted =.52), spelling (F(12,32)=4.06; p=.009); R 2 Adjusted =.45), and calculation (F(12,32)=4.47; p=.002); R 2 Adjusted =.57).
Higher digit span forward scores significantly predicted better spelling (β=.37; t(44)=2.55; p=.004) and calculation (β=.42; t(44)=3.04; p=.005) performance. Of note is that for reading, no individual variable contribution was significant, yet the contributions of both digit span forward and digit span backward approached significance (β=.35; t(44)=2.42; p=.02, and β=.34; t(44)=2.21; p=.03, respectively). Thus, overall, higher digit span forward predicted better academic performance even when considering the contribution of all executive and demographic variables (Figure 2)
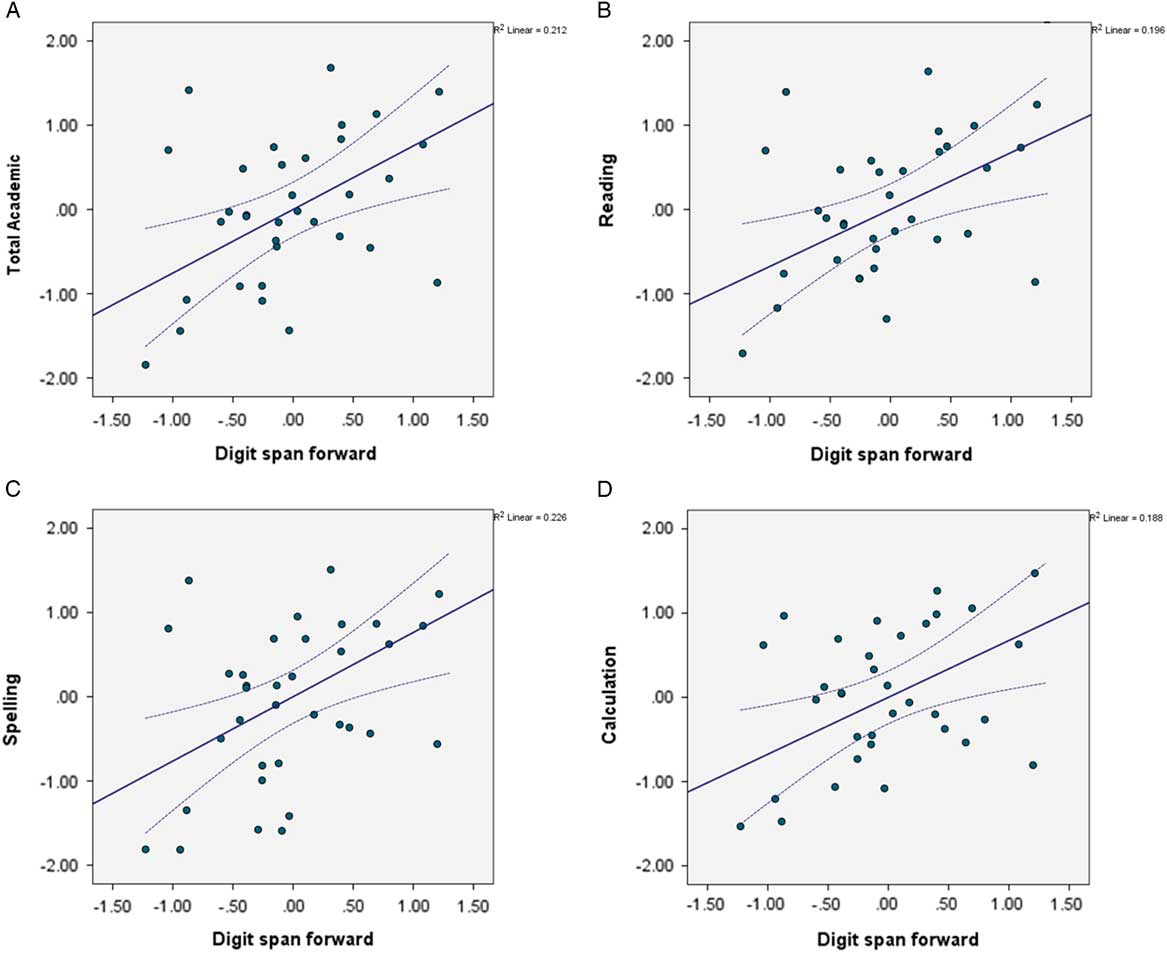
Fig. 2 Partial regression plot for digit span forward. Higher digit span forward, better academic performance across subject areas: (A) total academic achievement, (B) reading, (C), spelling (D) calculation.
DISCUSSION
The goal of this study was to help clarify understanding about academic performance in children with dystrophinopathy. This was done by examining academic functioning and the association with dystrophin gene mutation position and by determining if known executive weaknesses (selective attention/inhibitory control, set-shifting, working memory, processing speed) contribute significantly to academic performance, when studied in combination with motor limitations, intellectual function, behavior, SES, and mutation position.
As expected, and consistent with our previous finding, academic skills are reduced in children with dystrophinopathy. Even though the mean difference from IQ across subject areas was less than a standard deviation and, therefore, not great enough to meet criteria for a specific learning disorder, the finding of reduced academic performance nonetheless may represent significant difficulties for these children in real world functioning. These findings extend and replicate our prior research (Hinton et al., Reference Hinton, De Vivo, Fee, Goldstein and Stern2004) indicating that academics are overall weak across subject areas for children with dystrophinopathy.
Our findings also revealed, as predicted, that individuals with more downstream dystrophin gene mutations performed more poorly across academic measures. Overall mean performance across subject areas was significantly lower for those with mutations downstream of exon 43 as compared to average performance for those upstream. These results are consistent with those showing IQ is lower in individuals with mutations downstream of exon 43 (Daoud et al., Reference Daoud, Angeard, Demerre, Martie, Benyaou, Leturcq and Tuffery2009; Rasic et al., Reference Rasic, Vojinovic, Pesovic, Mijalkovic, Lukic, Mladenovic and Romac2014; Taylor et al., Reference Taylor, Betts, Maroulis, Gilissen, Pedersen, Mowat and Buckley2010; Wingeier et al., Reference Wingeier, Giger, Strozzi, Kreis, Joncourt, Conrad and Steinlin2011), as is digit span performance (Ricotti et al., Reference Ricotti, Mandy, Scoto, Pane, Deconinck, Messina and Muntoni2016; Fee et al., 2017, in submission). Thus, mutations that disrupt the dystrophin isoforms Dp140 and Dp 71 affect academic performance.
Our work examined multiple things that may contribute to real world academic function. Interestingly, our regression model revealed that among boys with dystrophinopathy, academic achievement was best predicted by performance on digits forward even when potential contributing factors including IQ, SES, illness severity, mutation position, and behavior were entered in the model. Digits forward was not only predicative of total academic achievement, but also predicted performance in spelling and calculation. Moreover, there was a trend toward digits forward also contributing to reading. Performance on other tests of executive functioning did not contribute significantly, contrary to our initial hypothesis.
Our current results replicate our previous studies that found that digit span contributed to academic performance in children with dystrophinopathy (Hinton et al., Reference Hinton, De Vivo, Fee, Goldstein and Stern2004) and digit span (both forward and backward span) contributed to the variance in reading performance in a large cohort of children with dystrophinopathy (Leaffer et al., Reference Leaffer, Fee and Hinton2016). Unlike those studies, the current study extended the investigation by examining the contribution of a variety of executive skills, as measured by individual performance and parent ratings, on academic performance. Although children with dystrophinopathy have generalized weakness across executive functions (Fee et al., 2017, in submission), surprisingly, only digit span forward significantly predicted the variance observed on academic tests.
Performance on digits forward likely reflects individual capacity embedded in several more complex abilities including executive and academic tasks. The finding that forward digit span, rather than IQ or the other variables, accounted for the largest amount of statistical variance is valuable knowledge for real world functioning. In academics, research has shown that increased load imposes large demands on working memory and thus limited capacity in working memory results in poor academic performance (Gathercole et al., Reference Gathercole, Pickering, Knight and Stegmann2004). Working memory has been demonstrated to be a strong predictor of academic achievement (Alloway & Alloway, Reference Alloway and Alloway2010). True deficits in the dystrophinopathies are likely an interaction of weakness in multiple areas including specific aspects of executive functioning. Learning is incremental, and difficulty in the acquisition of basic concepts that depend on linguistic capacity may result in academic failure as material becomes more complex.
We have proposed in a series of publications that the core cognitive deficit in the dystrophinopathy group is a limited verbal span. This hypothesis is based on a repeated pattern of poor performance on digit span, weak story memory, and impaired sentence repetition found in our studies suggesting a reduced capacity in linguistic load (Hinton et al., Reference Hinton, De Vivo, Nereo, Goldstein and Stern2001; Hinton, Fee, Goldstein, & De Vivo, Reference Hinton, Fee, Goldstein and De Vivo2007). It is also based on the finding that even across intellectual level, performance on tasks of digit span and story memory remains selectively low for children with dystrophinopathy (Hinton, De Vivo, Nereo, Goldstein, & Stern, Reference Hinton, De Vivo, Nereo, Goldstein and Stern2000). We expanded our investigation of verbal span and found that both digits forward and backward was poor demonstrating that decreased span as well as working memory was deficient. The combined deficits in verbal span and executive control was found to contribute to poor reading performance (Leaffer et al., Reference Leaffer, Fee and Hinton2016).
More recently, we challenged our assumption of a core deficit in verbal span, and found that across executive skills, children with dystrophinopathy have a generalized weakness in both cognitive and behavioral executive functioning (Fee et al., 2017, in submission). Yet, despite finding generalized executive deficits, only limited forward span was associated with mutation position (Fee et al., 2017, in submission). The current study again challenged the verbal span hypothesis by predicting that generalized executive deficits would contribute significant variance to academic performance, yet the results found that only forward span predicted academic performance. Taken together, the combined findings of digit span forward being the only executive skill to be associated with mutation position and the only executive skill to contribute significantly to academic performance support the hypothesis of a distinct weakness in the working memory system of individuals with dystrophinopathy. Both executive deficits and limitations in linguistic load are likely part of that system. Together, they reflect an underlying core deficit in verbal span in this group.
The strengths of the current study lie in the design to examine academic outcomes that represent real world functioning. Academics not only measure knowledge based learning but also reflect individual potential for success in future goals. Individual abilities were examined across academic areas to better isolate the source of cognitive weakness. The current study examined a wide range of executive skills in the context of several individual variables including physical functioning and demographics to decipher underlying contribution to performance. We chose a variety of psychometrically strong measures controlling for several potential confounds to delineate potential deficits in specific aspects of functioning. The finding of one specific deficit, performance on digits forward, in the midst of so many potential contributing factors, including IQ, physical abilities, behavior, and demographic factors, predicting academic performance emphasizes the strength of the finding.
Additionally, this study is the first to examine molecular associations with real world academic performance. This not only aids in the understanding of the relationship between a disorder with a known molecular cause and selective cognitive impairments, but also a better understanding of the various contributions to academic achievement. Finally, the sample included individuals from diverse backgrounds, which may be more representative of the dystrophinopathy population than found in many other cognitive studies. The found correlation between frequency of subjects with a downstream mutation and family income in our sample may further support other data demonstrating the significant impact of SES on cognitive development (Bradley & Corwyn, Reference Bradley and Corwyn2002) and may be indicative of a gene–environment interaction that should be further explored within this population.
The study also has several limitations. The study used a relatively small sample by including only those with fully completed measures. Although the sample size was deemed adequate and produced valid findings, a larger sample for the regression model might provide more robust results and greater confidence in conclusions. Another potential weakness was the lack of a control group. The sample’s performance was compared to normative data; however, each participant was examined as his own control strengthening the internal validity of the study. Finally, our academics measures assessed basic skills of language and arithmetic; more complex academic measures would help to further describe the extent and specifics of academic impairment within the population. Future studies could expand on our results and examine other measures that examine these cognitive constructs further.
This study contributes to further understanding the role of disrupted dystrophin in the brain and its impact on functioning. In addition, the study provides information that could aid in improving real world functioning for children with dystrophinopathy. Weaknesses in working memory disrupts everyday activities, particularly in the academic environment that requires organization and goal-directed behavior. The identification of these specific areas of weakness provides targets for remediation that can help in the attainment of appropriate developmental gains promoting a better quality of life.
ACKNOWLEDGMENTS
We are very grateful to the families who took the time and effort to participate in our project. Special thanks to Sally Dunaway, PT, DPT, Ashwini K. Rao, OTR, EdD, Mercedes Vega Villar, Fiona McMahon, Ruta Patel, and Justine Payne for their contribution to the physical therapy assessments. This work was supported by a grant from the Muscular Dystrophy Association to V.J.H. Disclosures: Fee, R.J., Montes, J, Stewart, J.L., and Hinton, V.J. declare that they have no conflicts of interest.









