Micronutrient deficiencies affect about two billion people globally1. Of these deficiencies vitamin A, iron, zinc and iodine are still major public health problems in the developing world with vitamin C, D and B deficiencies having declined considerably in recent decadesReference Muller and Krawinkel2. The impact of micronutrient deficiencies is established early in life and leads to growth stunting, decreased cognitive abilities, greater severity and rates of infection and decreased output of both physical and mental capacityReference Demment, Young and Sensenig3.
Micronutrient deficiencies continue to contribute to the burden of morbidity and mortality in South Africa. A high prevalence (39%) of suboptimal vitamin A deficiency (serum retinol < 20 μg dl−1) was found in South African preschool children in 1994 in a nationally representative survey. Furthermore, 11% of children had a haemoglobin concentration of less than 11 g dl−1 and 25% had low iron stores (ferritin < 12 μg dl−1)4. Numerous dietary surveys have supported findings of micronutrient deficiencies in both childrenReference Labadarios, Steyn, Maunder, MacIntyre, Swart and Gericke5 and adultsReference Steyn, Burger, Monyeki, Alberts and Nthangeni6, Reference Grobbelaar, Le Roux, Lindenberg, Marais and Steyn7.
Results of a study to assess the attributable burden due to selected deficiencies estimates that, in 2000, about 3000 deaths in children aged below 4 years due to diarrhoea were attributed to vitamin A deficiency. Furthermore, 519 maternal deaths were attributed to vitamin A deficiency in pregnant women, while more than 3000 perinatal deaths were attributed to iron deficiency anaemia in 2000Reference Nojilana, Norman, Dhansay, van Stuijvenberg and Bradshaw8. No national biochemical data are available for adults. However, some localised studies have shown high prevalences of iron deficiency in pregnant and non-pregnant womenReference Kruger, Dhansay, Van Staden, Faber, Badenhorst and Mansvelt9–Reference Faber, Jogessar and Benadé11 while others did notReference Wolmarans, Dhansay, Mansvelt, Laubscher and BenadéB12. High levels of vitamin A deficiency, particularly in HIV-infected adultsReference Kennedy-Oji, Coutsoudis, Kuhn, Pillay, Mburu and Stein13, Reference Visser, Maartens, Kossew and Hussey14 also in women from a rural area in South Africa, have been reportedReference Faber, Jogessar and Benadé11.
In 1999, the first National Food Consumption Survey (NFCS) was undertaken in South Africa in 1- to 9-year-old childrenReference Labadarios, Steyn, Maunder, MacIntyre, Swart and Gericke5. This survey showed that many micronutrients, including calcium, iron, zinc, vitamin A, C and E, niacin, riboflavin, vitamin B6 and folate, were deficient in the diet, particularly in rural children. Isolated studies in adults have shown similar micronutrient deficiencies and support the findings in childrenReference Steyn, Burger, Monyeki, Alberts and Nthangeni6,Reference Grobbelaar, Le Roux, Lindenberg, Marais and Steyn7. The underlying reason for these deficiencies has been attributed to the fact that the most commonly consumed foods, namely maize, sugar, tea and bread, are not good sources of the deficient nutrientsReference Steyn, Nel, Nantel, Kennedy and Labadarios15.
One of the outcomes of the NFCS was the initiation of a national mandatory food fortification programme by the Department of Health. Since maize meal and bread were the most commonly consumed staple foods, it was decided to use them as vehicles for fortification. Hence, vitamin A, iron, zinc, folic acid, thiamine, niacin, vitamin B6 and riboflavin have been added to maize meal and wheat flour in South Africa since October 200316. This was considered to be a sustainable and relatively inexpensive way to eradicate vitamin and mineral deficiencies without changes in traditional food consumption patternsReference Kotiah17. However, the effectiveness of this mandatory fortification process in the dosage supplied to the average South African adult has not been evaluated. The research questions of this study relate to the micronutrient adequacy of the dietary intakes of adult South Africans and the possible impact of mandatory fortification of maize meal and wheat flour on the average micronutrient intake of adults.
Methods
Since there has never been a national dietary survey of adults in South Africa, secondary data analysis was conducted on existing dietary databases (raw data) obtained from surveys undertaken in South Africa between 1983 and 2000Reference Steyn, Nel and Casey18. Data were extrapolated from surveys on adults and the following databases were utilised to differing degrees: Lebowa Study (black rural, 1–25 years; n = 483)Reference Badenhorst, Steyn, Jooste, Nel, Kruger and Oelofse19; Dikgale Study (black rural adults 19–60 years; n = 209)Reference Steyn, Burger, Monyeki, Alberts and Nthangeni20; Black Risk Factor Study (BRISK) (black urban 3–60+ years; n = 1507)Reference Bourne, Langenhoven, Steyn, Jooste, Laubscher and Van der Vyfer21; Transition Health and Urbanisation Study (THUSA) (black urban and rural adults 18 years and older; n = 890)Reference MacIntyre, Kruger, Venter and Vorster22; THUSA Bana Study (black urban and rural children 6–18 years; n = 1257)23; First Year Female Student (FYFS) Project (black urban and rural women 18–34 years; n = 136)Reference Steyn, Senekal, Brits and Nel24; Weight and Risk Factor Study (WRFS) (adults 18 years and older from all ethnic groups; n = 449)Reference Steyn, Senekal, Brits, Alberts, Mashego and Nel25; the Coronary Risk Factor Study (CORIS) (white adults 15 years and older; n = 1784)26; and the NFCSReference Labadarios, Steyn, Maunder, MacIntyre, Swart and Gericke5 data (children from all ethnic groups; n = 2868).
Dietary intakes from studies that used the 24-hour recall method were combined to produce a single adult estimate, using ratios between urban and rural intakes per province as calculated from the 1996 census data to generate a composite ‘average’ South African intake for males and females. The weighting of these data sets gives proportional representation of children aged 15 years and older (n = 3229). The 1996 census data were used because these results were validated against the NFCSReference Labadarios, Steyn, Maunder, MacIntyre, Swart and Gericke5, which used the sampling framework of the 1996 census.
Validations of results of the combined data set were carried out as follows. First, the NFCSReference Labadarios, Steyn, Maunder, MacIntyre, Swart and Gericke5 data were utilised since this study was conducted nationally in all nine provinces, and can be seen as representing South African children as a whole. The NFCS data were divided into 18 strata (nine provinces: urban and rural within each province), and each stratum was divided into two age groups, i.e. 1–5 and 6–9 years. Intakes, measured by per capita consumption and percentage in group consuming various items in each of these 36 cohorts (18 × 2 age groups), were correlated with each of the different data sets reported aboveReference Badenhorst, Steyn, Jooste, Nel, Kruger and Oelofse19–26 with similar ages and geographical regions, where available. Children’s data (per capita intake and percentage consuming an item) were also correlated with adult data in similar geographical regions. Results showed clear correlations among NFCS children’s data and adult data sets within similar regions27.
The next step was to determine the relationship between intakes of different cohorts, because of a lack of adult data sets representing all cohorts. Factor analyses, using food items consumed as observations and per capita consumption per cohort (as well as percentage consuming the food items per cohort), were used to establish relationships between the 18 cohorts. Results showed clearly that cohorts with similar population distribution characteristics (with regard to race and urban/rural distribution) had similar food consumption patterns27.
Having established that relationships exist between different cohorts and also between NFCS data and the adult data sets per cohort led to a combined database being generated using weights described above, to give proportional representation of South Africans of 15 years and older (n = 3229)27.
The final step was to establish measures of validity and reliability for the food lists created by the weighed estimates of the original databases. Concurrent validity was established by comparing intakes with those of the Food and Agricultural Organization’s (FAO) food balance sheets27, and further, with intakes calculated for corresponding items in other countries. Reliability was determined by comparing adult data with that of the NFCS data in order to ascertain that a smooth, logical flow from ‘children’ consumption to ‘adult’ consumption was achieved27.
Comprehensive detail of the methodology is presented in the publication ‘Report on Food Consumption Studies Undertaken Amongst Different Population Groups (1983–2000). Average Intakes of Foods Most Commonly Consumed’27.
Calculation of nutrient adequacy ratios (NARs)
In order to determine the micronutrient adequacy of the diet, an NARReference Hatloy, Torheim and Oshaug28 was calculated for each micronutrient: vitamin A, B6, B12, C, niacin, thiamine, riboflavin and minerals calcium, iron and zinc. NAR was calculated as the ratio of the intake of a nutrient divided by the recommended intake for that nutrient (RNI), using World Health Organization/FAO recommended intakes29, which are set at 2 standard deviations above the average requirements. The RNI have been recommended for use in developing countries since they provide options on cut-off levels depending on bioavailability with regard to nutrients such as iron and zinc when the diet is predominantly plant- and/or legume-based. In the case of iron and zinc, the category for moderate bio-availability was used in the present study. The mean adequacy ratio (MAR) was calculated as the measure of adequacy of the overall diet: MAR = sum of each NAR (truncated at 1)/number of nutrientsReference Hatloy, Torheim and Oshaug28. For both NAR and MAR, a value of 1.0 or 100% is the ideal, since it means that the intake is the same as the requirement.
The micronutrient intake of the participants, as calculated by secondary data analyses, was re-calculated by substituting the nutrient values for maize meal porridge and bread with values from analysed fortified samples of maize meal porridgeReference Wolmarans, Danster and Chetty30 and fortified white and brown bread, currently available in the market (N Vorster, personal communication). Table 1 provides the nutrient values per 100 g of unfortified and fortified maize meal porridge, white and brown bread, used in the calculations. The percentage of persons consuming maize meal and wheat flour was calculated and the average amount consumed per person or per capita were also calculated.
Table 1 Mean values (per 100 g) for cooked stiff maize, meal porridgeFootnote * and bread† with and without fortification
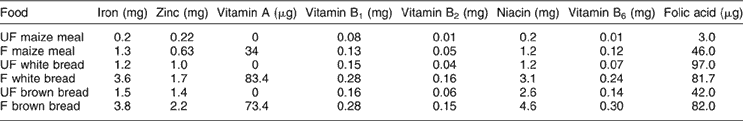
UF – unfortified food as per original dietary survey; F – fortified food.
* Wolmarans et al. 30.
† Nonnie Voster, personal communication, previously from Pioneer Foods South Africa.
Data analyses
Data were analysed using SAS System for Windows, Release 8.02 (SAS Institute Inc.). The dietary databases used in this study made use of the Medical Research Council Food Composition Tables and software31.
Results
With regard to micronutrients and minerals (Table 2), men had mean calcium (557.6 mg), folate (281.6 μg), riboflavin (1.2 mg) and vitamin B6 (1.2 mg) intakes less than 75% RNI, while the remaining nutrients were closer or greater than 100% RNI. In women, the intakes were far poorer. Both calcium (410.3 mg) and iron (8.8 mg) lay below 50% RNI, while folate (231.4) and vitamin B6 (0.9 mg) were less than 75% RNI. The only mean intakes above 100% RNI in women were zinc, vitamin A, C and vitamin B12. The poorer quality of the women’s diet is also reflected by their MAR of 60% compared with 66% for men.
Table 2 Mean micronutrient intake of South African adults as derived from secondary dietary analysis
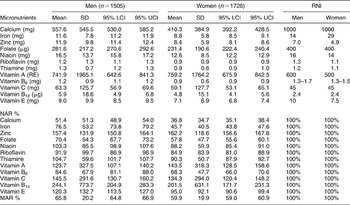
SD – standard deviation; LCI – lower confidence interval; UCI – upper confidence interval; RNI – recommended nutrient intake; NAR – nutrient adequacy ratio, calculated as intake/RNI × 100%; MAR – mean adequacy ratio, calculated as sum of RNIs/no. of nutrients.
Table 3 presents data for urban and rural areas. There are large differences between the two areas, with urban areas having a better-quality diet overall than that of rural areas (MAR 67.5% vs. 57%). In urban areas, only mean calcium (593.4 mg), iron (10.5 mg) and folate (265.1 μg) intakes fall below 75% RNI. In rural areas, mean calcium (343.4 mg), iron (9.5 mg), folate (243.4 μg), vitamin B6 (0.9 mg) and vitamin E (6.1 mg) levels are low.
Table 3 Mean micronutrient intake of South African adults by urban and rural distribution

SD – standard deviation; LCI – lower confidence interval; UCI – upper confidence interval; RNI – recommended nutrient intake; NAR – nutrient adequacy ratio, calculated as intake/RNI × 100%; MAR – mean adequacy ratio, calculated as sum of RNIs/no. of nutrients.
Table 4 shows the outcomes of fortification on the mean nutrient intakes of males and females. In males, mean nutrient intakes increased to at/above the RNIs for all nutrients with the exception of folate in urban areas. In women, in urban areas, thiamine, folate and iron intakes remained below the RNIs, while in rural areas only iron remained below the RNI. Furthermore, there was an overall improvement in MAR, which increased in males and females and in urban and rural areas. Fortification increased MAR in men from 65.8% to 79.9% and in women from 59.9% to 72.7%.
Table 4 Mean micronutrient intakes of South African adults with fortifiedFootnote * maize and bread substituted for the unfortified products

UF – unfortified; F – fortified; WHO – World Health Organization; RNI – recommended nutrient intake; MAR – mean adequacy ratio, calculated as sum of RNIs/no. of nutrients.
* Fortification mix does not include calcium, vitamin C and vitamin E.
† Mean value lies above the RNI.
Table 5 presents data on the intake of staple food items. It should be noted that the percentage and per capita intake of maize meal porridge (858 g) and bread/fat cakes (147.1 g) are considerably higher in rural areas than in urban areas. This accounts for the greater improvements in mean nutrient intakes in the rural areas as a result of fortification.
Table 5 Intake of staple foods (maize and bread) by South Africans (n = 3229)
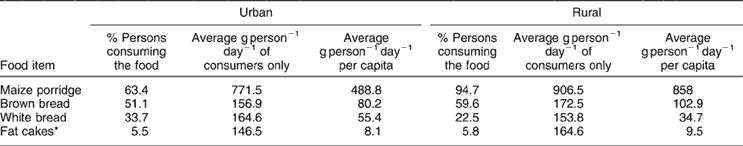
* Round pieces of bread dough deep-fried in oil.
Discussion
The study has certain limitations that need to be acknowledged. The results generated are from the combined database which is not based on a national survey of adults. Furthermore, the studies included in the database were undertaken over different time periods, specifically the study on white adults, which was undertaken in the late 1970s. It should also be recognised that most of these studies were undertaken using a single 24-hour recall, which places further questions on the validity of the data since the 24-hour recall has been reported to underreport nutrient intake.32 However, since there are no national data on adult South Africans, we believe that the present data fill a gap and provide some trends, provided the limitations are recognised and acknowledged. Furthermore, the data can be utilised to illustrate the benefits of fortification.
Overall the micronutrient content of the diet of adult South Africans appeared to be far from optimal and reflected one having numerous deficiencies. Since the black population comprises 79% of the population of South Africa33, the overall dietary intake resulting from the secondary data analyses mainly reflects their eating pattern. The large majority of the African population still lives on a low income of less than 10 dollars a day; only 20.4% have grade 12 or higher education level and 33.7% are unemployed33.
The data resulting from this study showed that four nutrients were particularly low in the diet of adults; namely calcium, iron, folate and vitamin B6. In children studied in the NFCSReference Labadarios, Steyn, Maunder, MacIntyre, Swart and Gericke5, the most deficient micronutrients were vitamin A, calcium, iron, zinc, folate, vitamin B6, niacin, riboflavin and vitamin C and E. Hence, deficiencies of calcium, iron, folate and vitamin B6 appear to be common to both adults and children. Furthermore, in the NFCS micronutrient intakes were generally far lower in rural areas, which was also the case in the present study. This would most probably be due to the fact that the rural areas in South Africa are more economically and socially deprived than the urban ones33.
Berner et al.Reference Berner, Clydesdale and Douglass34 evaluated the effects of food fortification on the diet of Americans older than 1 year. In a similar process to the present study, the researchers theoretically calculated micronutrient values of food, with and without fortification. In total, 246 food items were found to be fortified, most of these being in a breakfast cereal category (183) or in a fortified beverage (38); 21 meal replacements and supplementation and four calcium-fortified beverages. Median intakes for the population as a whole (with fortification) exceeded recommended dietary allowances (RDA) with the exception of vitamin A, calcium, iron and zinc. Furthermore, vitamin C and folate would also have been below RDA if not fortified.
To be effective and safe, fortification should reach people in need while at the same time not contributing to excessive intakesReference Berner, Clydesdale and Douglass34. Hence, policy-makers and the food industry need to have updated and reliable data on what the population consumes in order to introduce fortification at a national level. Langenhoven et al.Reference Langenhoven, Wolmarans, Jooste, Dhansay and Benadé35 showed that maize meal and bread are the main staple foods of South Africans, hence their fortification will benefit the majority of the population. This was shown to be true for the present study, since fortification of two main staple foods made significant improvement to the adults’ micronutrient intakes. Furthermore, the greatest improvements were found in rural areas where they are most needed. This demonstrates how government policies can have a major beneficial effect on dietary intake and ultimately on nutritional health.
However, the benefits of fortification may not reach everyone. There are areas in South Africa where people do not eat bread or maize meal on a regular basis. For example, on some farms in the Western Cape Province, workers and their families are supplied with potatoes, since these grow on the farm and are accessible and available to workers as a source of staple food.Reference Grobbelaar, Le Roux, Lindenberg, Marais and Steyn7 In Durban, there is a large Asian population who mainly consume rice.Reference Wolmarans, Seedat, Mayet, Joubert and Wentzel36 It is thus necessary to realise that South Africa is very diverse with regard to its ethnic and cultural groups and this influences dietary patterns. Not all groups consume maize meal as a staple food, and wheat (bread) consumption may be less than expected when starches such as potatoesReference Grobbelaar, Le Roux, Lindenberg, Marais and Steyn7 or riceReference Wolmarans, Seedat, Mayet, Joubert and Wentzel36 are available as an economical option to bread.
Yip and RamakrishnanReference Yip and Ramakrishnan37 have highlighted the issues that need to be addressed regarding fortification, particularly in developing countries. The first of these is a lack of data that demonstrate the effectiveness of fortification and the second is the need for good monitoring systems. In South Africa, both of these issues are of concern. Mandatory iodation of salt was introduced nationally in 1995. However, results from an iodine-deficiency surveyReference Immelman, Towindo and Kalk38 in 1998 in primary-school children showed that there were up to 42% of schools in certain provinces where children were found to be iodine deficient. The reasons proposed for this situation were the fact that 6.5% of households used non-iodised salt (meant for agriculture) and further that there was under or non-iodation of a substantial percentage of household salt. Hence, there needs to be continuous monitoring to ensure the effectiveness of the fortification process in terms of the stability of the products from factory to household. How effectively the mandatory fortification of wheat and maize is monitored is still not clear.
Furthermore, fortification in the South African scenario does not solve all micronutrient deficiencies. Some nutrients like calcium may not be present in the fortification mix, while others like iron may possibly not meet the high demands for iron, particularly by women of childbearing age. It is therefore prudent to follow other options for improving the micronutrient quality of diet in the long term, namely supplementation (of iron and vitamin A), diversification and nutrition educationReference Yip and Ramakrishnan37. A supplementation programme of iron and folate to pregnant women and vitamin A in children has been running for a number of years in South Africa. With regard to education, the Department of Health has demonstrated their concern for the nutritional health of the population by developing and implementing food-based dietary guidelines for South AfricaReference Vorster, Love and Browne39. These include the following guidelines aimed at improving micronutrient intake, particularly of iron, zinc and calcium: ‘Foods from animals can be eaten every day’ and ‘Eat dry beans, split peas and soya regularly’ and to improve vitamin A, folate and vitamin C: ‘Eat plenty of vegetables and fruit every day’.
Conclusion
The average diet of South African adults was low in iron, calcium, folate and vitamin B6, particularly in women and in rural areas. Fortification of maize meal and wheat flour (bread) considerably improved mean vitamin B6, thiamine, riboflavin, niacin, folate, zinc and iron intakes and increased the overall MAR of the diet.









